The fluorescent pentameric oligothiophene pFTAA identifies filamentous tau in live neurons cultured from adult P301S tau mice
- PMID: 26074756
- PMCID: PMC4448042
- DOI: 10.3389/fnins.2015.00184
The fluorescent pentameric oligothiophene pFTAA identifies filamentous tau in live neurons cultured from adult P301S tau mice
Abstract
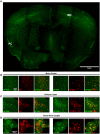
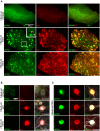
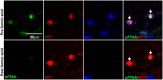
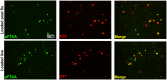
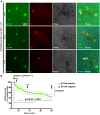
Identification of fluorescent dyes that label the filamentous protein aggregates characteristic of neurodegenerative disease, such as β-amyloid and tau in Alzheimer's disease, in a live cell culture system has previously been a major hurdle. Here we show that pentameric formyl thiophene acetic acid (pFTAA) fulfills this function in living neurons cultured from adult P301S tau transgenic mice. Injection of pFTAA into 5-month-old P301S tau mice detected cortical and DRG neurons immunoreactive for AT100, an antibody that identifies solely filamentous tau, or MC1, an antibody that identifies a conformational change in tau that is commensurate with neurofibrillary tangle formation in Alzheimer's disease brains. In fixed cultures of dorsal root ganglion (DRG) neurons, pFTAA binding, which also identified AT100 or MC1+ve neurons, followed a single, saturable binding curve with a half saturation constant of 0.14 μM, the first reported measurement of a binding affinity of a beta-sheet reactive dye to primary neurons harboring filamentous tau. Treatment with formic acid, which solubilizes filamentous tau, extracted pFTAA, and prevented the re-binding of pFTAA and MC1 without perturbing expression of soluble tau, detected using an anti-human tau (HT7) antibody. In live cultures, pFTAA only identified DRG neurons that, after fixation, were AT100/MC1+ve, confirming that these forms of tau pre-exist in live neurons. The utility of pFTAA to discriminate between living neurons containing filamentous tau from other neurons is demonstrated by showing that more pFTAA+ve neurons die than pFTAA-ve neurons over 25 days. Since pFTAA identifies fibrillar tau and other misfolded proteins in living neurons in culture and in animal models of several neurodegenerative diseases, as well as in human brains, it will have considerable application in sorting out disease mechanisms and in identifying disease-modifying drugs that will ultimately help establish the mechanisms of neurodegeneration in human neurodegenerative diseases.
Keywords: Alzheimer's disease; dorsal root ganglion neurons; fluorescent vital fibrillar tau dye; frontotemporal dementia (FTD); hyperphosphorylated tau; neurofibrillary tangles; transgenic P301S tau mouse.
Figures

Comment in
-
Labeling Pathological Tau: An Important Quest for the Unknown.Front Neurosci. 2016 Jun 6;10:253. doi: 10.3389/fnins.2016.00253. eCollection 2016. Front Neurosci. 2016. PMID: 27377198 Free PMC article. No abstract available.
References
-
- Aslund A., Sigurdson C. J., Klingstedt T., Grathwohl S., Bolmont T., Dickstein D. L., et al. . (2009). Novel pentameric thiophene derivatives for in vitro and in vivo optical imaging of a plethora of protein aggregates in cerebral amyloidoses. ACS Chem. Biol. 4, 673–684. 10.1021/cb900112v - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

