REM Sleep at its Core - Circuits, Neurotransmitters, and Pathophysiology
- PMID: 26074874
- PMCID: PMC4448509
- DOI: 10.3389/fneur.2015.00123
REM Sleep at its Core - Circuits, Neurotransmitters, and Pathophysiology
Abstract
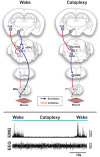
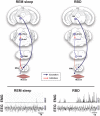
Rapid eye movement (REM) sleep is generated and maintained by the interaction of a variety of neurotransmitter systems in the brainstem, forebrain, and hypothalamus. Within these circuits lies a core region that is active during REM sleep, known as the subcoeruleus nucleus (SubC) or sublaterodorsal nucleus. It is hypothesized that glutamatergic SubC neurons regulate REM sleep and its defining features such as muscle paralysis and cortical activation. REM sleep paralysis is initiated when glutamatergic SubC cells activate neurons in the ventral medial medulla, which causes release of GABA and glycine onto skeletal motoneurons. REM sleep timing is controlled by activity of GABAergic neurons in the ventrolateral periaqueductal gray and dorsal paragigantocellular reticular nucleus as well as melanin-concentrating hormone neurons in the hypothalamus and cholinergic cells in the laterodorsal and pedunculo-pontine tegmentum in the brainstem. Determining how these circuits interact with the SubC is important because breakdown in their communication is hypothesized to underlie narcolepsy/cataplexy and REM sleep behavior disorder (RBD). This review synthesizes our current understanding of mechanisms generating healthy REM sleep and how dysfunction of these circuits contributes to common REM sleep disorders such as cataplexy/narcolepsy and RBD.
Keywords: REM sleep; REM sleep behavior disorder; amygdala; brainstem; cataplexy; dopamine; hypothalamus; narcolepsy.
Figures
References
-
- Kryger MH, Roth T, Dement WC. Principles and Practice of Sleep Medicine. 5th ed Philadelphia, PA: Saunders/Elsevier; (2011).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

