The Novel Functions of High-Molecular-Mass Complexes Containing Insulin Receptor Substrates in Mediation and Modulation of Insulin-Like Activities: Emerging Concept of Diverse Functions by IRS-Associated Proteins
- PMID: 26074875
- PMCID: PMC4443775
- DOI: 10.3389/fendo.2015.00073
The Novel Functions of High-Molecular-Mass Complexes Containing Insulin Receptor Substrates in Mediation and Modulation of Insulin-Like Activities: Emerging Concept of Diverse Functions by IRS-Associated Proteins
Abstract
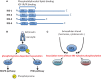
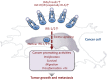
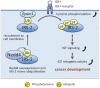
Insulin-like peptides, such as insulin-like growth factors (IGFs) and insulin, induce a variety of bioactivities, such as growth, differentiation, survival, increased anabolism, and decreased catabolism in many cell types and in vivo. In general, IGFs or insulin bind to IGF-I receptor (IGF-IR) or insulin receptor (IR), activating the receptor tyrosine kinase. Insulin receptor substrates (IRSs) are known to be major substrates of receptor kinases, mediating IGF/insulin signals to direct bioactivities. Recently, we discovered that IRSs form high-molecular-mass complexes (referred to here as IRSomes) even without IGF/insulin stimulation. These complexes contain proteins (referred to here as IRSAPs; IRS-associated proteins), which modulate tyrosine phosphorylation of IRSs by receptor kinases, control IRS stability, and determine intracellular localization of IRSs. In addition, in these complexes, we found not only proteins that are involved in RNA metabolism but also RNAs themselves. Thus, IRSAPs possibly contribute to modulation of IGF/insulin bioactivities. Since it is established that disorder of modulation of insulin-like activities causes various age-related diseases including cancer, we could propose that the IRSome is an important target for treatment of these diseases.
Keywords: IRS-associated protein; IRSome; cancer; insulin; insulin receptor substrate; insulin-like growth factor.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

