Effect of in-feed administration and withdrawal of tylosin phosphate on antibiotic resistance in enterococci isolated from feedlot steers
- PMID: 26074889
- PMCID: PMC4444845
- DOI: 10.3389/fmicb.2015.00483
Effect of in-feed administration and withdrawal of tylosin phosphate on antibiotic resistance in enterococci isolated from feedlot steers
Abstract
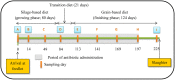
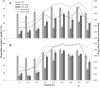
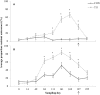
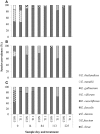
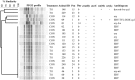
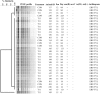
Tylosin phosphate is a macrolide commonly administered to cattle in North America for the control of liver abscesses. This study investigated the effect of in-feed administration of tylosin phosphate to cattle at subtherapeutic levels and its subsequent withdrawal on macrolide resistance using enterococci as an indicator bacterium. Fecal samples were collected from steers that received no antibiotics and steers administered tylosin phosphate (11 ppm) in-feed for 197 days and withdrawn 28 days before slaughter. Enterococcus species isolated from fecal samples were identified through sequencing the groES-EL intergenic spacer region and subject to antimicrobial susceptibility testing, identification of resistance determinants and pulsed-field gel electrophoresis profiling. Tylosin increased (P < 0.05) the proportion of ery(R) and tyl(R) enterococci within the population. Just prior to its removal, the proportion of ery(R) and tyl(R) resistant enterococci began decreasing and continued to decrease after tylosin was withdrawn from the diet until there was no difference (P > 0.05) between treatments on d 225. This suggests that antibiotic withdrawal prior to slaughter contributes to a reduction in the proportion of macrolide resistant enterococci entering the food chain. Among the 504 enterococci isolates characterized, Enterococcus hirae was found to predominate (n = 431), followed by Enterococcus villorum (n = 32), Enterococcus faecium (n = 21), Enterococcus durans (n = 7), Enterococcus casseliflavus (n = 4), Enterococcus mundtii (n = 4), Enterococcus gallinarum (n = 3), Enterococcus faecalis (n = 1), and Enterococcus thailandicus (n = 1). The diversity of enterococci was greater in steers at arrival than at exit from the feedlot. Erythromycin resistant isolates harbored the erm(B) and/or msrC gene. Similar PFGE profiles of ery(R) E. hirae pre- and post-antibiotic treatment suggest that increased abundance of ery(R) enterococci after administration of tylosin phosphate reflects selection for strains that were already present within the gastrointestinal tract of cattle at arrival.
Keywords: antimicrobial resistance; beef cattle; enterococci; erythromycin; subtherapeutic macrolides; tylosin.
Figures

References
-
- Alexander T. W., Reuter T., Sharma R., Yanke L. J., Topp E., McAllister T. A. (2009). Longitudinal characterization of resistant Escherichia coli in fecal deposits from cattle fed subtherapeutic levels of antimicrobials. Appl. Environ. Microbiol. 75, 7125–7134. 10.1128/AEM.00944-09 - DOI - PMC - PubMed
-
- Alexander T. W., Yanke L. J., Topp E., Olson M. E., Read R. R., Morck D. W., et al. . (2008). Effect of subtherapeutic administration of antibioitics on the prevalence of antibiotic-resistant Escherichia coli bacteria in feedlot cattle. Appl. Environ. Microbiol. 74, 4405–4416. 10.1128/AEM.00489-08 - DOI - PMC - PubMed
-
- Alfouzan W., Al-Sheriday S., Al-Jabban A., Dhar R., Al-Mutairi A. R., Udo E. (2014). A case of multiple splenic abscesses due to Enterococcus hirae. JMM Case Rep. 1, 3 10.1099/jmmcr.0.001214 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

