Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: a comprehensive review
- PMID: 26075266
- PMCID: PMC4446472
- DOI: 10.1155/2015/814068
Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: a comprehensive review
Abstract
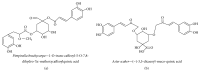
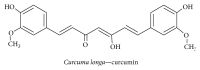
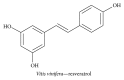
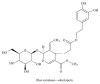
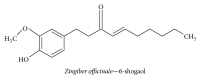
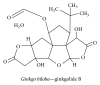
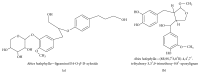
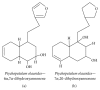
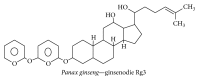
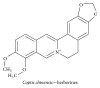
Alzheimer's disease (AD), characterized by progressive dementia and deterioration of cognitive function, is an unsolved social and medical problem. Age, nutrition, and toxins are the most common causes of AD. However, currently no credible treatment is available for AD. Traditional herbs and phytochemicals may delay its onset and slow its progression and also allow recovery by targeting multiple pathological causes by antioxidative, anti-inflammatory, and antiamyloidogenic properties. They also regulate mitochondrial stress, apoptotic factors, free radical scavenging system, and neurotrophic factors. Neurotrophins such as BDNF, NGF, NT3, and NT4/5 play a vital role in neuronal and nonneuronal responses to AD. Neurotrophins depletion accelerates the progression of AD and therefore, replacing such neurotrophins may be a potential treatment for neurodegenerative disease. Here, we review the phytochemicals that mediate the signaling pathways involved in neuroprotection specifically neurotrophin-mediated activation of Trk receptors and members of p75(NTR) superfamily. We focus on representative phenolic derivatives, iridoid glycosides, terpenoids, alkaloids, and steroidal saponins as regulators of neurotrophin-mediated neuroprotection. Although these phytochemicals have attracted attention owing to their in vitro neurotrophin potentiating activity, their in vivo and clinical efficacy trials has yet to be established. Therefore, further research is necessary to prove the neuroprotective effects in preclinical models and in humans.
Figures

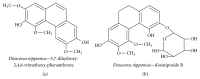
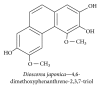
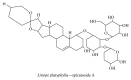

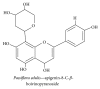




References
-
- Rajput A. H. Frequency and cause of Parkinson's disease. The Canadian Journal of Neurological Sciences. 1992;19(1, supplement):103–107. - PubMed
-
- Harper P. S. The epidemiology of Huntington's disease. Human Genetics. 1992;89(4):365–376. - PubMed
-
- Kurtzke J. F. Epidemiology of amyotrophic lateral sclerosis. Advances in Neurology. 1982;36:281–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

