Broad-spectrum antivirals against viral fusion
- PMID: 26075364
- PMCID: PMC4554337
- DOI: 10.1038/nrmicro3475
Broad-spectrum antivirals against viral fusion
Abstract
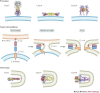
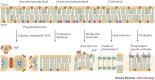
Effective antivirals have been developed against specific viruses, such as HIV, Hepatitis C virus and influenza virus. This 'one bug-one drug' approach to antiviral drug development can be successful, but it may be inadequate for responding to an increasing diversity of viruses that cause significant diseases in humans. The majority of viral pathogens that cause emerging and re-emerging infectious diseases are membrane-enveloped viruses, which require the fusion of viral and cell membranes for virus entry. Therefore, antivirals that target the membrane fusion process represent new paradigms for broad-spectrum antiviral discovery. In this Review, we discuss the mechanisms responsible for the fusion between virus and cell membranes and explore how broad-spectrum antivirals target this process to prevent virus entry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Lee, B. & Rota, P. A. (eds) Henipavirus: Ecology, Molecular Virology and Pathogenesis (Springer, 2012).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

