PHD2/3-dependent hydroxylation tunes cardiac response to β-adrenergic stress via phospholamban
- PMID: 26075818
- PMCID: PMC4563684
- DOI: 10.1172/JCI80369
PHD2/3-dependent hydroxylation tunes cardiac response to β-adrenergic stress via phospholamban
Abstract
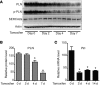
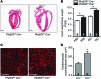
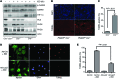
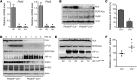
Ischemic heart disease is the leading cause of heart failure. Both clinical trials and experimental animal studies demonstrate that chronic hypoxia can induce contractile dysfunction even before substantial ventricular damage, implicating a direct role of oxygen in the regulation of cardiac contractile function. Prolyl hydroxylase domain (PHD) proteins are well recognized as oxygen sensors and mediate a wide variety of cellular events by hydroxylating a growing list of protein substrates. Both PHD2 and PHD3 are highly expressed in the heart, yet their functional roles in modulating contractile function remain incompletely understood. Here, we report that combined deletion of Phd2 and Phd3 dramatically decreased expression of phospholamban (PLN), resulted in sustained activation of calcium/calmodulin-activated kinase II (CaMKII), and sensitized mice to chronic β-adrenergic stress-induced myocardial injury. We have provided evidence that thyroid hormone receptor-α (TR-α), a transcriptional regulator of PLN, interacts with PHD2 and PHD3 and is hydroxylated at 2 proline residues. Inhibition of PHDs increased the interaction between TR-α and nuclear receptor corepressor 2 (NCOR2) and suppressed Pln transcription. Together, these observations provide mechanistic insight into how oxygen directly modulates cardiac contractility and suggest that cardiac function could be modulated therapeutically by tuning PHD enzymatic activity.
Figures




References
-
- Schomig A. Adrenergic mechanisms in myocardial infarction: cardiac and systemic catecholamine release. J Cardiovasc Pharmacol. 1988;12(suppl 1):S1–S7. - PubMed
-
- Kushner FG, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120(22):2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

