Differential signaling mechanism for HIV-1 Nef-mediated production of IL-6 and IL-8 in human astrocytes
- PMID: 26075907
- PMCID: PMC4467202
- DOI: 10.1038/srep09867
Differential signaling mechanism for HIV-1 Nef-mediated production of IL-6 and IL-8 in human astrocytes
Abstract
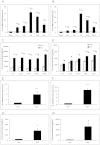
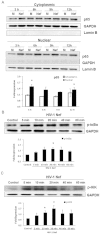
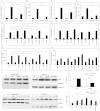
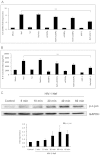
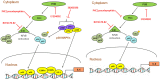
Variety of HIV-1 viral proteins including HIV-1 Nef are known to activate astrocytes and microglia in the brain and cause the release of pro-inflammatory cytokines, which is thought to be one of the mechanisms leading to HIV-1- mediated neurotoxicity. IL-6 and IL-8 have been found in the CSF of patients with HIV-1 associated dementia (HAD), suggesting that they might play important roles in HIV-1 neuropathology. In the present study we examined the effects of HIV-1 Nef on IL-6 and IL-8 induction in astrocytes. The results demonstrate that both IL-6 and IL-8 are significantly induced in HIV-1 Nef-transfected SVGA astrocytes and HIV-1 Nef-treated primary fetal astrocytes. We also determined the molecular mechanisms responsible for the HIV-1 Nef-induced increased IL-6 and IL-8 by using chemical inhibitors and siRNAs against PI3K/Akt/PKC, p38 MAPK, NF-κB, CEBP and AP-1. Our results clearly demonstrate that the PI3K/PKC, p38 MAPK, NF-κB and AP-1 pathways are involved in HIV-1 Nef-induced IL-6 production in astrocytes, while PI3K/PKC and NF-κB pathways are involved in HIV-1 Nef-induced IL-8 production. These results offer new potential targets to develop therapeutic strategy for treatment of HIV-1 associated neurological disorders, prevalent in > 40% of individuals infected with HIV-1.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



References
-
- McArthur J. C. HIV dementia: an evolving disease. J Neuroimmunol 157, 3–10 (2004). - PubMed
-
- Ances B. M. & Ellis R. J. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol 27, 86–92 (2007). - PubMed
-
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis 186 Suppl 2, S193–198 (2002). - PubMed
-
- Norenberg M. D. Astrocyte responses to CNS injury. J Neuropathol Exp Neurol 53, 213–220 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

