Similar Occurrence of Febrile Episodes Reported in Non-Atopic Children at Three to Five Years of Age after Prebiotics Supplemented Infant Formula
- PMID: 26076141
- PMCID: PMC4468127
- DOI: 10.1371/journal.pone.0129927
Similar Occurrence of Febrile Episodes Reported in Non-Atopic Children at Three to Five Years of Age after Prebiotics Supplemented Infant Formula
Abstract
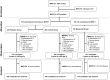
This is a follow up study of a multicenter randomised placebo-controlled trial in seven centres in five West European countries. The RCT assessed the effect of infant formula supplemented with a mixture of prebiotics (with neutral short-chain and long-chain oligosaccharides and pectin-derived acidic oligosaccharides) during infancy in term-born children (n=1130). In the follow-up study 672 children (60% of the study population) participated: 232 (56%) from the prebiotics group (PG), 243 (58%) from the control group (CG), and 197 (66%) from the non-randomised breast-fed group (BG). The primary outcome was the occurrence of febrile episodes at three to five years of age prospectively documented by the parents: in the PG 1.17 (interquartile range 0.50-2.08) episodes per year versus 1.20 (0.52-2.57) in the CG; and 1.48 (0.65-2.60) in the BG. This specific prebiotics mixture given during infancy in healthy non-atopic subjects does not decrease febrile episodes and therefore seems not to prevent infection between their third and fifth birthday.
Conflict of interest statement
References
-
- Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, et al. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr 2004;145:297–303. - PubMed
-
- Bakker-Zierikzee AM, Alles MS, Knol J, Kok FJ, Tolboom JJM, Bindels JG. Effect of infant formulas containing prebiotics or probiotics on the intestinal flora during the first months of life. J Nutr 2005;94:783–790. Dundee infant feeding study. - PubMed
-
- Eiwegger T, Stahl B, Schmitt J, Boehm G, Gerstmayr M, Pichler J, et al. Human milk derived oligosaccharides and plant derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr Res 2004;56: 536–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical