Octreotide s.c. depot provides sustained octreotide bioavailability and similar IGF-1 suppression to octreotide LAR in healthy volunteers
- PMID: 26076191
- PMCID: PMC4574831
- DOI: 10.1111/bcp.12698
Octreotide s.c. depot provides sustained octreotide bioavailability and similar IGF-1 suppression to octreotide LAR in healthy volunteers
Abstract
Aims: The aim was to assess the pharmacokinetics, pharmacodynamics, safety and tolerability of octreotide subcutaneous (s.c.) depot, a novel octreotide formulation.
Methods: This was a phase I, randomized, open label study. After a single dose of octreotide immediate release (IR) 200 µg, subjects were randomized to one of eight groups to receive three monthly injections of octreotide s.c. depot A 10, 20 or 30 mg, B 30 mg, C 10, 20 or 30 mg or long acting octreotide (octreotide LAR) 30 mg.
Results: One hundred and twenty-two subjects were randomized. For all depot variants, onset of octreotide release was rapid and sustained for up to 4 weeks. The relative octreotide bioavailability of depot variants vs. octreotide IR ranged from 0.68 (90% confidence interval [CI] 0.61, 0.76) to 0.91 (90% CI 0.81, 1.02) and, vs. octreotide LAR, was approximately four- to five-fold greater: 3.97 (90% CI 3.35, 4.71) to 5.27 ng ml(-1) h (90% CI 4.43, 6.27). All depot variants showed relatively rapid initial reductions of insulin-like growth factor 1 (IGF-1) compared with octreotide LAR. A trend of octreotide dose dependence was also indicated from the plasma concentrations and suppression of IGF-1. Maximum inhibition of IGF-1 at steady-state was highest for depot B and C. All depot treatments were well tolerated. The most frequent adverse events were gastrointestinal related.
Conclusions: Octreotide s.c. depot provides greater octreotide bioavailability with a more rapid onset and stronger suppression of IGF-1 than octreotide LAR in healthy volunteers.
Keywords: FluidCrystal®; depot; formulation; injection; octreotide; subcutaneous.
© 2015 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures
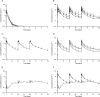
 , octreotide s.c. depot A, 10 mg;
, octreotide s.c. depot A, 10 mg;  , octreotide s.c. depot A, 20 mg;
, octreotide s.c. depot A, 20 mg;  , octreotide s.c. depot A, 30 mg;
, octreotide s.c. depot A, 30 mg;  , octreotide s.c. depot B, 30 mg;
, octreotide s.c. depot B, 30 mg;  , octreotide s.c. depot C, 10 mg;
, octreotide s.c. depot C, 10 mg;  , octreotide s.c. depot C, 20 mg;
, octreotide s.c. depot C, 20 mg;  , octreotide s.c. depot C, 30 mg;
, octreotide s.c. depot C, 30 mg;  , octreotide LAR, 30 mg), B) octreotide s.c. depot A (
, octreotide LAR, 30 mg), B) octreotide s.c. depot A ( , octreotide s.c. depot A, 10 mg;
, octreotide s.c. depot A, 10 mg;  , octreotide s.c. depot A, 20 mg;
, octreotide s.c. depot A, 20 mg;  , octreotide s.c. depot A, 30 mg), C) octreotide s.c. depot B (
, octreotide s.c. depot A, 30 mg), C) octreotide s.c. depot B ( , octreotide s.c. depot B, 30 mg), D) octreotide s.c. depot C (
, octreotide s.c. depot B, 30 mg), D) octreotide s.c. depot C ( , octreotide s.c. depot C, 10 mg;
, octreotide s.c. depot C, 10 mg;  , octreotide s.c. depot C, 20 mg;
, octreotide s.c. depot C, 20 mg;  , octreotide s.c. depot C, 30 mg), E) octreotide LAR (
, octreotide s.c. depot C, 30 mg), E) octreotide LAR ( , octreotide LAR, 30 mg) and F) octreotide s.c. depot B superimposed with octreotide LAR (
, octreotide LAR, 30 mg) and F) octreotide s.c. depot B superimposed with octreotide LAR ( , octreotide s.c. depot B, 30 mg;
, octreotide s.c. depot B, 30 mg;  , octreotide LAR, 30 mg)
, octreotide LAR, 30 mg)
 , octreotide s.c. depot A, 10 mg;
, octreotide s.c. depot A, 10 mg;  , octreotide s.c. depot A, 20 mg;
, octreotide s.c. depot A, 20 mg;  , octreotide s.c. depot A, 30 mg;
, octreotide s.c. depot A, 30 mg;  , octreotide s.c. depot B, 30 mg;
, octreotide s.c. depot B, 30 mg;  , octreotide s.c. depot C, 10 mg;
, octreotide s.c. depot C, 10 mg;  , octreotide s.c. depot C, 20 mg;
, octreotide s.c. depot C, 20 mg;  , octreotide s.c. depot C, 30 mg;
, octreotide s.c. depot C, 30 mg;  , octreotide LAR, 30 mg), B) octreotide s.c. depot A (
, octreotide LAR, 30 mg), B) octreotide s.c. depot A ( , octreotide s.c. depot A, 10 mg;
, octreotide s.c. depot A, 10 mg;  , octreotide s.c. depot A, 20 mg;
, octreotide s.c. depot A, 20 mg;  , octreotide s.c. depot A, 30 mg), C) octreotide s.c. depot B (
, octreotide s.c. depot A, 30 mg), C) octreotide s.c. depot B ( , octreotide s.c. depot B, 30 mg), D) octreotide s.c. depot C (
, octreotide s.c. depot B, 30 mg), D) octreotide s.c. depot C ( , octreotide s.c. depot C, 10 mg;
, octreotide s.c. depot C, 10 mg;  , octreotide s.c. depot C, 20 mg;
, octreotide s.c. depot C, 20 mg;  , octreotide s.c. depot C, 30 mg), E) octreotide LAR (
, octreotide s.c. depot C, 30 mg), E) octreotide LAR ( , octreotide LAR, 30 mg) and F) octreotide s.c. depot B superimposed with octreotide LAR (
, octreotide LAR, 30 mg) and F) octreotide s.c. depot B superimposed with octreotide LAR ( , octreotide s.c. depot B, 30 mg;
, octreotide s.c. depot B, 30 mg;  , octreotide LAR, 30 mg)
, octreotide LAR, 30 mg)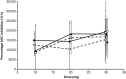
 , Oct s.c. depot A, first dose;
, Oct s.c. depot A, first dose;  , Oct s.c. depot B, first dose;
, Oct s.c. depot B, first dose;  , Oct s.c. depot C, first dose;
, Oct s.c. depot C, first dose;  , Oct LAR, first dose;
, Oct LAR, first dose;  , Oct s.c. depot A, third dose;
, Oct s.c. depot A, third dose;  , Oct s.c. depot B, third dose;
, Oct s.c. depot B, third dose;  , Oct s.c. depot C, third dose;
, Oct s.c. depot C, third dose;  , Oct LAR, third dose
, Oct LAR, third doseReferences
-
- Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly - 2011 update. Endocr Pract. 2011;17(Suppl 4):1–44. - PubMed
-
- Pavel M, Kidd M, Modlin I. Systemic therapeutic options for carcinoid. Semin Oncol. 2013;40:84–99. - PubMed
-
- Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557–77. - PMC - PubMed
-
- National Comprehensive Cancer Network, Inc. 2006. NCCN Practice Guidelines in Oncology™ Neuroendocrine Tumors (Version 2.0). Available at: http://www.nccn.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

