Targeted Lipid Profiling Discovers Plasma Biomarkers of Acute Brain Injury
- PMID: 26076478
- PMCID: PMC4468135
- DOI: 10.1371/journal.pone.0129735
Targeted Lipid Profiling Discovers Plasma Biomarkers of Acute Brain Injury
Abstract
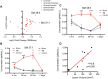
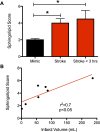
Prior efforts to identify a blood biomarker of brain injury have relied almost exclusively on proteins; however their low levels at early time points and poor correlation with injury severity have been limiting. Lipids, on the other hand, are the most abundant molecules in the brain and readily cross the blood-brain barrier. We previously showed that certain sphingolipid (SL) species are highly specific to the brain. Here we examined the feasibility of using SLs as biomarkers for acute brain injury. A rat model of traumatic brain injury (TBI) and a mouse model of stroke were used to identify candidate SL species though our mass-spectrometry based lipid profiling approach. Plasma samples collected after TBI in the rat showed large increases in many circulating SLs following injury, and larger lesions produced proportionately larger increases. Plasma samples collected 24 hours after stroke in mice similarly revealed a large increase in many SLs. We constructed an SL score (sum of the two SL species showing the largest relative increases in the mouse stroke model) and then evaluated the diagnostic value of this score on a small sample of patients (n = 14) who presented with acute stroke symptoms. Patients with true stroke had significantly higher SL scores than patients found to have non-stroke causes of their symptoms. The SL score correlated with the volume of ischemic brain tissue. These results demonstrate the feasibility of using lipid biomarkers to diagnose brain injury. Future studies will be needed to further characterize the diagnostic utility of this approach and to transition to an assay method applicable to clinical settings.
Conflict of interest statement
Figures


References
-
- Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. (2007) Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 115: e478–e534. 10.1161/CIRCULATIONAHA.107.181486 - DOI - PubMed
-
- Del Zoppo GJ, Saver JL, Jauch EC, Adams HP, American Heart Association Stroke Council (2009) Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation 40: 2945–2948. 10.1161/STROKEAHA.109.192535 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

