CES1 genetic variation affects the activation of angiotensin-converting enzyme inhibitors
- PMID: 26076923
- PMCID: PMC6329299
- DOI: 10.1038/tpj.2015.42
CES1 genetic variation affects the activation of angiotensin-converting enzyme inhibitors
Abstract
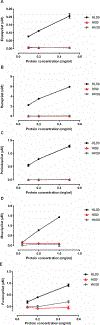
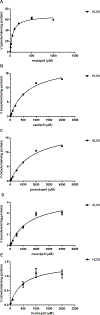
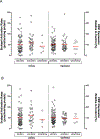
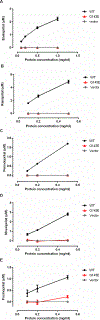
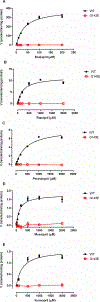
The aim of the study was to determine the effect of carboxylesterase 1 (CES1) genetic variation on the activation of angiotensin-converting enzyme inhibitor (ACEI) prodrugs. In vitro incubation study of human liver, intestine and kidney s9 fractions demonstrated that the ACEI prodrugs enalapril, ramipril, perindopril, moexipril and fosinopril are selectively activated by CES1 in the liver. The impact of CES1/CES1VAR and CES1P1/CES1P1VAR genotypes and diplotypes on CES1 expression and activity on enalapril activation was investigated in 102 normal human liver samples. Neither the genotypes nor the diplotypes affected hepatic CES1 expression and activity. Moreover, among several CES1 nonsynonymous variants studied in transfected cell lines, the G143E (rs71647871) was a loss-of-function variant for the activation of all ACEIs tested. The CES1 activity on enalapril activation in human livers with the 143G/E genotype was approximately one-third of that carrying the 143G/G. Thus, some functional CES1 genetic variants (for example, G143E) may impair ACEI activation, and consequently affect therapeutic outcomes of ACEI prodrugs.
Conflict of interest statement
Conflict of interests
No conflict of interests was declared.
Figures



References
-
- Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375(9718): 906–915. - PubMed
-
- Tsoukas G, Anand S, Yang K. Dose-dependent antihypertensive efficacy and tolerability of perindopril in a large, observational, 12-week, general practice-based study. Am J Cardiovasc Drugs 2011; 11(1): 45–55. - PubMed
-
- Malacco E, Omboni S, Volpe M, Auteri A, Zanchetti A. Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly patients with mild to moderate essential hypertension: the ESPORT study. J Hypertens 2010; 28(11): 2342–2350. - PubMed
-
- Ionescu DD. Antihypertensive efficacy of perindopril 5–10 mg/day in primary health care: an open-label, prospective, observational study. Clin Drug Investig 2009; 29(12): 767–776. - PubMed
-
- Mugellini A, Dobovisek J, Planinc D, Cremonesi G, Fogari R. Efficacy and safety of delapril plus manidipine compared with enalapril plus hydrochlorothiazide in mild to moderate essential hypertension: results of a randomized trial. Clin Ther 2004; 26(9): 1419–1426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

