Subgingival microbiome in patients with healthy and ailing dental implants
- PMID: 26077225
- PMCID: PMC4468443
- DOI: 10.1038/srep10948
Subgingival microbiome in patients with healthy and ailing dental implants
Abstract
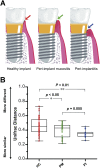
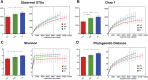
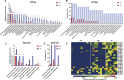
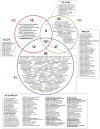
Dental implants are commonly used to replace missing teeth. However, the dysbiotic polymicrobial communities of peri-implant sites are responsible for peri-implant diseases, such as peri-implant mucositis and peri-implantitis. In this study, we analyzed the microbial characteristics of oral plaque from peri-implant pockets or sulci of healthy implants (n = 10), peri-implant mucositis (n = 8) and peri-implantitis (n = 6) sites using pyrosequencing of the 16S rRNA gene. An increase in microbial diversity was observed in subgingival sites of ailing implants, compared with healthy implants. Microbial co-occurrence analysis revealed that periodontal pathogens, such as Porphyromonas gingivalis, Tannerella forsythia, and Prevotella intermedia, were clustered into modules in the peri-implant mucositis network. Putative pathogens associated with peri-implantitis were present at a moderate relative abundance in peri-implant mucositis, suggesting that peri-implant mucositis an important early transitional phase during the development of peri-implantitis. Furthermore, the relative abundance of Eubacterium was increased at peri-implantitis locations, and co-occurrence analysis revealed that Eubacterium minutum was correlated with Prevotella intermedia in peri-implantitis sites, which suggests the association of Eubacterium with peri-implantitis. This study indicates that periodontal pathogens may play important roles in the shifting of healthy implant status to peri-implant disease.
Figures


References
-
- Jepsen S., Ruhling A., Jepsen K., Ohlenbusch B. & Albers H. K. Progressive peri-implantitis. Incidence and prediction of peri-implant attachment loss. Clin Oral Implants Res 7, 133–142 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

