Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis
- PMID: 26077231
- PMCID: PMC4477037
- DOI: 10.1038/ncomms8375
Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis
Abstract
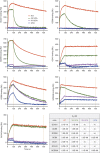
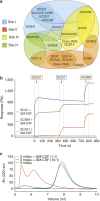
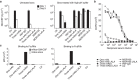
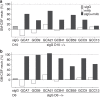
Pulmonary alveolar proteinosis (PAP) is a severe autoimmune disease caused by autoantibodies that neutralize GM-CSF resulting in impaired function of alveolar macrophages. In this study, we characterize 21 GM-CSF autoantibodies from PAP patients and find that somatic mutations critically determine their specificity for the self-antigen. Individual antibodies only partially neutralize GM-CSF activity using an in vitro bioassay, depending on the experimental conditions, while, when injected in mice together with human GM-CSF, they lead to the accumulation of a large pool of circulating GM-CSF that remains partially bioavailable. In contrast, a combination of three non-cross-competing antibodies completely neutralizes GM-CSF activity in vitro by sequestering the cytokine in high-molecular-weight complexes, and in vivo promotes the rapid degradation of GM-CSF-containing immune complexes in an Fc-dependent manner. Taken together, these findings provide a plausible explanation for the severe phenotype of PAP patients and for the safety of treatments based on single anti-GM-CSF monoclonal antibodies.
Conflict of interest statement
A.L. is the scientific founder of Humabs BioMed, a company that develops human antibodies for treatment of infectious diseases. D.C. is CSO of Humabs BioMed. A.L. and F.S. hold shares in Humabs BioMed. The remaining authors declare no competing financial interests.
Figures

References
-
- Watanabe M., Uchida K., Nakagaki K., Trapnell B. C. & Nakata K. High avidity cytokine autoantibodies in health and disease: pathogenesis and mechanisms. Cytokine Growth Factor Rev. 21, 263–273 (2010). - PubMed
-
- Madariaga L. et al. Detection of anti-interferon-gamma autoantibodies in subjects infected by Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2, 62–68 (1998). - PubMed
-
- Patel S. Y. et al. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J. Immunol. 175, 4769–4776 (2005). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

