Long-Term Follow-Up of the E1199 Phase III Trial Evaluating the Role of Taxane and Schedule in Operable Breast Cancer
- PMID: 26077235
- PMCID: PMC4500829
- DOI: 10.1200/JCO.2015.60.9271
Long-Term Follow-Up of the E1199 Phase III Trial Evaluating the Role of Taxane and Schedule in Operable Breast Cancer
Abstract
Purpose: To determine long-term outcomes in a clinical trial evaluating the role of taxane type and schedule in operable breast cancer and evaluate the impact of obesity and black race on outcome.
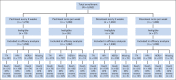
Patients and methods: A total of 4,954 eligible women with stage II to III breast cancer treated with four cycles of doxorubicin plus cyclophosphamide were randomly assigned to receive paclitaxel or docetaxel every 3 weeks for four doses or weekly for 12 doses using a 2 × 2 factorial design. The primary end point was disease-free survival (DFS). Results are expressed as hazard ratios (HRs) from Cox proportional hazards models. All P values are two sided.
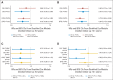
Results: When compared with the standard every-3-week paclitaxel arm, after a median follow-up of 12.1 years, DFS significantly improved and overall survival (OS) marginally improved only for the weekly paclitaxel (HR, 0.84; P = .011 and HR, 0.87; P = .09, respectively) and every-3-week docetaxel arms (HR, 0.79; P = .001 and HR, 0.86; P = .054, respectively). Weekly paclitaxel improved DFS and OS (HR, 0.69; P = .010 and HR, 0.69; P = .019, respectively) in triple-negative breast cancer. For hormone receptor-positive, human epidermal growth factor receptor 2-nonoverexpressing disease, no experimental arm improved OS, and black race and obesity were associated with increased risk of breast cancer recurrence and death.
Conclusion: Improved outcomes initially observed for weekly paclitaxel were qualitatively similar but quantitatively less pronounced with longer follow-up, although exploratory analysis suggested substantial benefit in triple-negative disease. Further research is required to understand why obesity and race influence clinical outcome in hormone receptor-positive disease.
Trial registration: ClinicalTrials.gov NCT00004125.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures




Comment in
-
Optimizing Adjuvant Taxanes in Early Breast Cancer.J Clin Oncol. 2015 Jul 20;33(21):2334-6. doi: 10.1200/JCO.2015.61.9312. Epub 2015 Jun 22. J Clin Oncol. 2015. PMID: 26101243 No abstract available.
References
-
- Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. - PubMed
-
- Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. - PubMed
-
- Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CA180867/CA/NCI NIH HHS/United States
- CA180795/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180795/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- CA180794/CA/NCI NIH HHS/United States
- U10 CA180864/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
- UG1 CA190140/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- UG1 CA189859/CA/NCI NIH HHS/United States
- CA180820/CA/NCI NIH HHS/United States
- CA180802/CA/NCI NIH HHS/United States
- CA190140/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- CA180790/CA/NCI NIH HHS/United States
- CA189859/CA/NCI NIH HHS/United States
- CA180888/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- CA180816/CA/NCI NIH HHS/United States
- CA180844/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- CA180864/CA/NCI NIH HHS/United States
- UG1 CA233184/CA/NCI NIH HHS/United States
- CA180821/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180816/CA/NCI NIH HHS/United States
- U10 CA180844/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

