Efficiency and safety of proprotein convertase subtilisin/kexin 9 monoclonal antibody on hypercholesterolemia: a meta-analysis of 20 randomized controlled trials
- PMID: 26077586
- PMCID: PMC4599534
- DOI: 10.1161/JAHA.115.001937
Efficiency and safety of proprotein convertase subtilisin/kexin 9 monoclonal antibody on hypercholesterolemia: a meta-analysis of 20 randomized controlled trials
Abstract
Background: Proprotein convertase subtilisin/kexin9 (PCSK9) monoclonal antibody significantly reduces low-density lipoprotein cholesterol level in patients with hypercholesterolemia. The goal of this study was to review recently reported randomized controlled trials to investigate the therapeutic effects and safety of PCSK9 inhibitors.
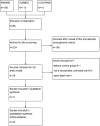
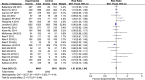
Methods and results: The clinical randomized controlled trials published from inception to March 19, 2015 were identified from The Cochrane Library databases, PUBMED, and EBASE. Randomized controlled trials of at least 8 weeks duration using PCSK9 inhibitors in treating patients with hypercholesterolemia were included. Mean difference (MD) with a 95% CI was used to calculate the continuous data, the standardized mean difference with a 95% CI was used when the unit was not unified, and risk ratio with a 95% CI was used for dichotomous data. After screening, 20 trials fulfilled the inclusion criteria. PCSK9 inhibitors significantly decreased the levels of low-density lipoprotein cholesterol (MD=-65.29 mg/dL, 95% CI: -72.08 to -58.49), total cholesterol (MD=-60.04 mg/dL, 95% CI: -69.95 to -50.13), triglycerides (MD=-12.21 mg/dL, 95% CI: -16.21 to -8.22) and apolipoprotein-B (MD=-41.01 mg/dL, 95% CI: -46.07 to -35.94), lipoprotein(a) (standardized mean difference=-0.94, 95% CI: -1.12 to -0.77) and increased the levels of high-density lipoprotein cholesterol (MD=3.40 mg/dL, 95% CI: 3.12 to 3.68) and apolipoprotein-A1 (MD=6.75 mg/dL, 95% CI: 4.64 to 8.86). There was no significant difference in the incidence of treatment-emergent adverse events (risk ratio=1.01, 95% CI: 0.98 to 1.04), serious treatment-emergent adverse events (risk ratio=1.01, 95% CI: 0.88 to 1.17), and the discontinuation of treatment between the 2 groups (risk ratio=1.07, 95% CI: 0.86 to 1.34).
Conclusions: The meta-analysis indicated that PCSK9 inhibitors had a strong effect in lowering low-density lipoprotein cholesterol and other lipid levels with satisfactory safety and tolerability in patients with hypercholesterolemia.
Keywords: lipids; lipoproteins; meta‐analysis; proprotein convertase subtilisin/kexin9 inhibitor.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures













References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. - PMC - PubMed
-
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. - PubMed
-
- Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel members. An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia—full report. J Clin Lipidol. 2014;8:29–60. - PubMed
-
- Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. - PubMed
-
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

