Interleukin-22 is increased in multiple sclerosis patients and targets astrocytes
- PMID: 26077779
- PMCID: PMC4480507
- DOI: 10.1186/s12974-015-0335-3
Interleukin-22 is increased in multiple sclerosis patients and targets astrocytes
Abstract
Background: Increasing evidences link T helper 17 (Th17) cells with multiple sclerosis (MS). In this context, interleukin-22 (IL-22), a Th17-linked cytokine, has been implicated in blood brain barrier breakdown and lymphocyte infiltration. Furthermore, polymorphism between MS patients and controls has been recently described in the gene coding for IL-22 binding protein (IL-22BP). Here, we aimed to better characterize IL-22 in the context of MS.
Methods: IL-22 and IL-22BP expressions were assessed by ELISA and qPCR in the following compartments of MS patients and control subjects: (1) the serum, (2) the cerebrospinal fluid, and (3) immune cells of peripheral blood. Identification of the IL-22 receptor subunit, IL-22R1, was performed by immunohistochemistry and immunofluorescence in human brain tissues and human primary astrocytes. The role of IL-22 on human primary astrocytes was evaluated using 7-AAD and annexin V, markers of cell viability and apoptosis, respectively.
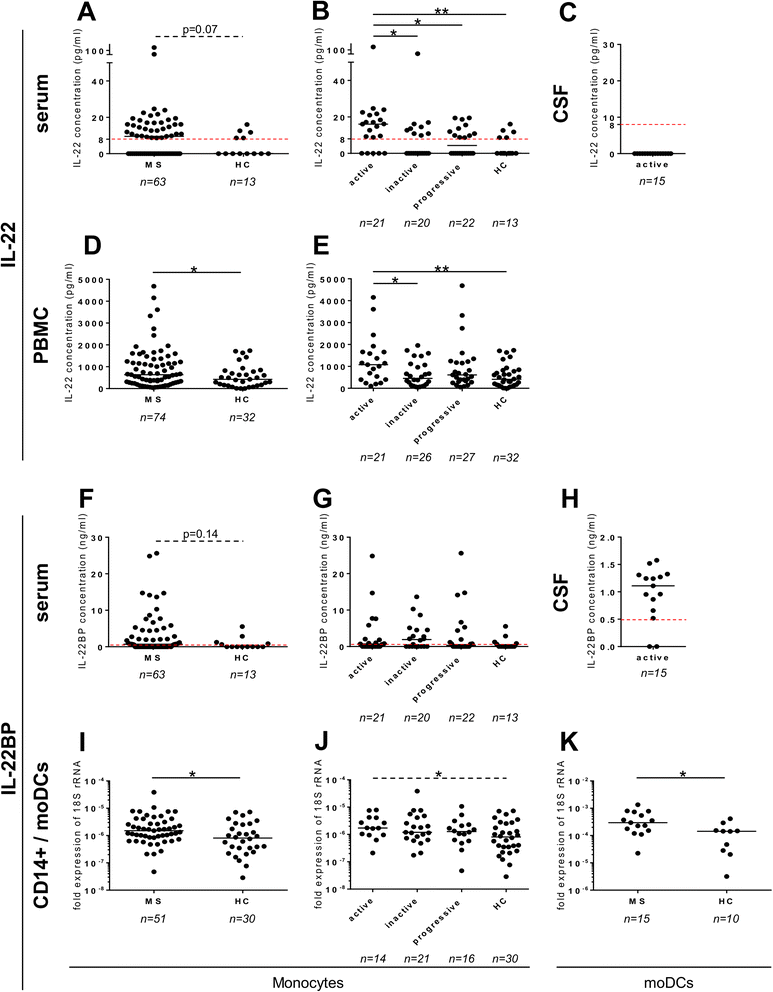
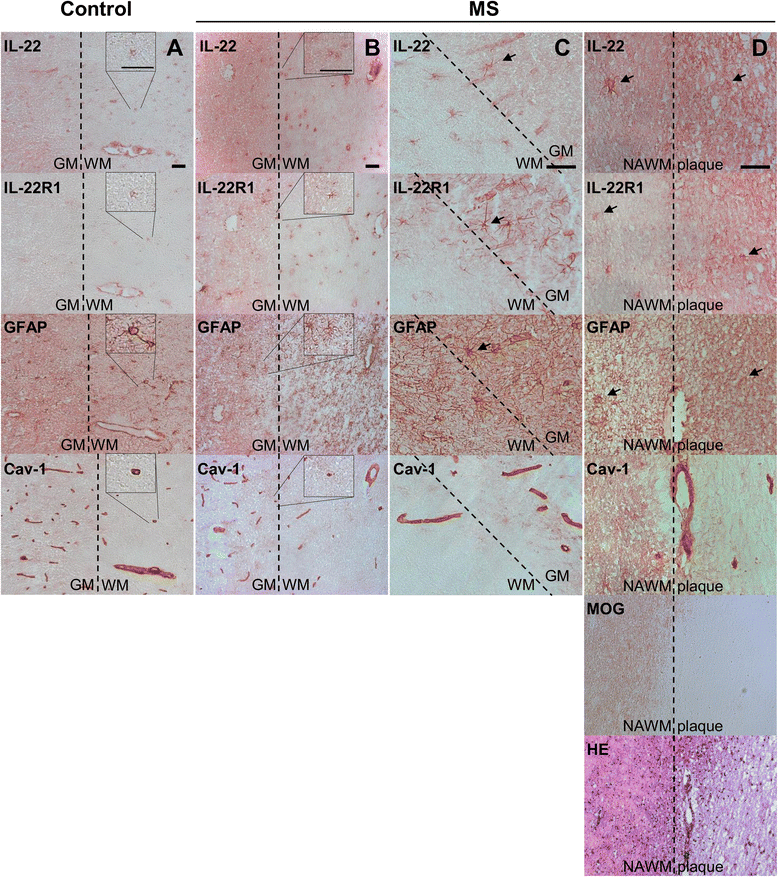
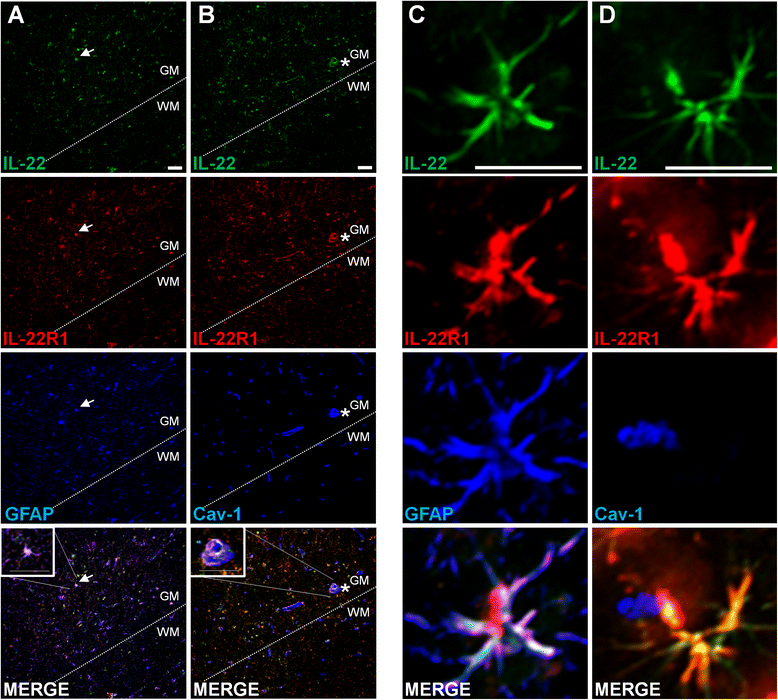
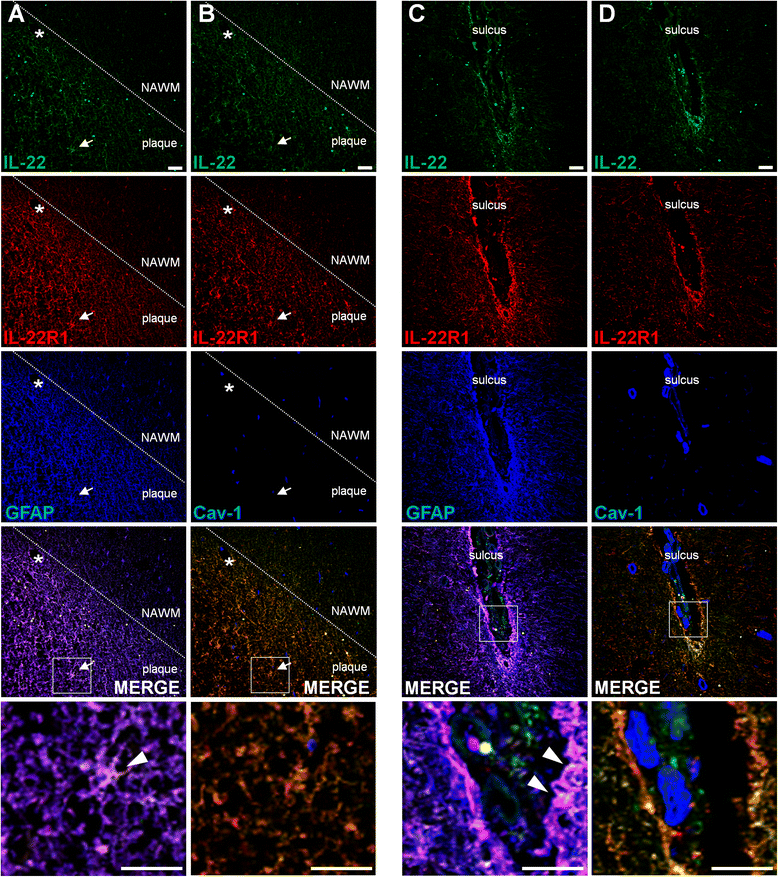
Results: In a cohort of 141 MS patients and healthy control (HC) subjects, we found that serum levels of IL-22 were significantly higher in relapsing MS patients than in HC but also remitting and progressive MS patients. Monocytes and monocyte-derived dendritic cells contained an enhanced expression of mRNA coding for IL-22BP as compared to HC. Using immunohistochemistry and confocal microscopy, we found that IL-22 and its receptor were detected on astrocytes of brain tissues from both control subjects and MS patients, although in the latter, the expression was higher around blood vessels and in MS plaques. Cytometry-based functional assays revealed that addition of IL-22 improved the survival of human primary astrocytes. Furthermore, tumor necrosis factor α-treated astrocytes had a better long-term survival capacity upon IL-22 co-treatment. This protective effect of IL-22 seemed to be conferred, at least partially, by a decreased apoptosis.
Conclusions: We show that (1) there is a dysregulation in the expression of IL-22 and its antagonist, IL-22BP, in MS patients, (2) IL-22 targets specifically astrocytes in the human brain, and (3) this cytokine confers an increased survival of the latter cells.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

