Enabling unassisted solar water splitting by iron oxide and silicon
- PMID: 26078190
- PMCID: PMC4490416
- DOI: 10.1038/ncomms8447
Enabling unassisted solar water splitting by iron oxide and silicon
Abstract
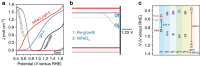
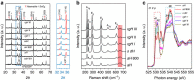
Photoelectrochemical (PEC) water splitting promises a solution to the problem of large-scale solar energy storage. However, its development has been impeded by the poor performance of photoanodes, particularly in their capability for photovoltage generation. Many examples employing photovoltaic modules to correct the deficiency for unassisted solar water splitting have been reported to-date. Here we show that, by using the prototypical photoanode material of haematite as a study tool, structural disorders on or near the surfaces are important causes of the low photovoltages. We develop a facile re-growth strategy to reduce surface disorders and as a consequence, a turn-on voltage of 0.45 V (versus reversible hydrogen electrode) is achieved. This result permits us to construct a photoelectrochemical device with a haematite photoanode and Si photocathode to split water at an overall efficiency of 0.91%, with NiFeOx and TiO2/Pt overlayers, respectively.
Figures


References
-
- Lewis N. S. Toward cost-effective solar energy use. Science 315, 798–801 (2007). - PubMed
-
- Daniel G. N. The artificial leaf. Acc. Chem. Res. 45, 767–776 (2012). - PubMed
-
- Blankenship R. E. et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809 (2011). - PubMed
-
- Fujishima A. & Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972). - PubMed
-
- Reece S. Y. et al. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334, 645–648 (2011). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

