Reversible Photoswitching of Carbon Dots
- PMID: 26078266
- PMCID: PMC4468586
- DOI: 10.1038/srep11423
Reversible Photoswitching of Carbon Dots
Abstract
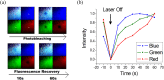
We present a method of reversible photoswitching in carbon nanodots with red emission. A mechanism of electron transfer is proposed. The cationic dark state, formed by the exposure of red light, is revived back to the bright state with the very short exposure of blue light. Additionally, the natural on-off state of carbon dot fluorescence was tuned using an electron acceptor molecule. Our observation can make the carbon dots as an excellent candidate for the super-resolution imaging of nanoscale biomolecules within the cell.
Figures


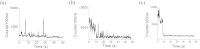

References
-
- Dickson R. M., Cubitt A. B., Tsien R. Y. & Moerner W. E. On/off blinking and switching behaviour of single molecules of green fluorescent protein. Nature 388, 355–358 (1997). - PubMed
-
- Betzig E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006). - PubMed
-
- Holzmeister P., Gietl A. & Tinnefeld P. Geminate recombination as a photoprotection mechanism for fluorescent dyes. Angew. Chem. Int. Ed. 53, 5685–5688 (2014). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

