Targeting protein arginine methyltransferase 5 inhibits colorectal cancer growth by decreasing arginine methylation of eIF4E and FGFR3
- PMID: 26078354
- PMCID: PMC4673200
- DOI: 10.18632/oncotarget.4332
Targeting protein arginine methyltransferase 5 inhibits colorectal cancer growth by decreasing arginine methylation of eIF4E and FGFR3
Abstract
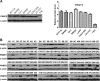
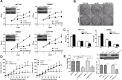
Protein arginine methyltransferases (PRMTs) plays critical roles in cancer. PRMT5 has been implicated in several types of tumors. However, the role of PRMT5 in cancer development remains to be fully elucidated. Here, we provide evidence that PRMT5 is overexpressed in colorectal cancer (CRC) cells and patient-derived primary tumors, correlated with increased cell growth and decreased overall patient survival. Arginine methyltransferase inhibitor 1 (AMI-1)strongly inhibited tumor growth, increased the ratio of Bax/Bcl-2, and induced apoptosis in mouse CRC xenograt model. AMI-1 also induced apoptosis and decreased the migratory activity in several CRC cells. In CRC xenografts AMI-1 significantly decreased symmetric dimethylation of histone 4 (H4R3me2s), a histone mark of type II PRMT5, but not the expression of H4R3me2a, a histone mark of type I PRMTs. These results suggest that the inhibition of PRMT5 contributes to the antitumor efficacy of AMI-1. Chromatin immunoprecipitation (ChIP) identified FGFR3 and eIF4E as two key genes regulated by PRMT5. PRMT5 knockdown reduced the levels of H4R3me2s and H3R8me2s methylation on FGFR3 and eIF4E promoters, leading to decreased expressions of FGFR3 and eIF4E. Collectively, our findings provide new evidence that PRMT5 plays an important role in CRC pathogenesis through epigenetically regulating arginine methylation of oncogenes such as eIF4E and FGFR3.
Keywords: AMI-1; FGFR3; PRMT5; colorectal cancer; eIF4E.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. - PubMed
-
- Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. - PubMed
-
- Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

