Remodeling of alveolar septa after murine pneumonectomy
- PMID: 26078396
- PMCID: PMC4587600
- DOI: 10.1152/ajplung.00042.2015
Remodeling of alveolar septa after murine pneumonectomy
Abstract
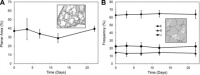
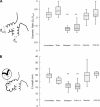
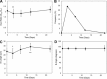
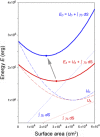
In most mammals, removing one lung (pneumonectomy) results in the compensatory growth of the remaining lung. In mice, stereological observations have demonstrated an increase in the number of mature alveoli; however, anatomic evidence of the early phases of alveolar growth has remained elusive. To identify changes in the lung microstructure associated with neoalveolarization, we used tissue histology, electron microscopy, and synchrotron imaging to examine the configuration of the alveolar duct after murine pneumonectomy. Systematic histological examination of the cardiac lobe demonstrated no change in the relative frequency of dihedral angle components (Ends, Bends, and Junctions) (P > 0.05), but a significant decrease in the length of a subset of septal ends ("E"). Septal retraction, observed in 20-30% of the alveolar ducts, was maximal on day 3 after pneumonectomy (P < 0.01) and returned to baseline levels within 3 wk. Consistent with septal retraction, the postpneumonectomy alveolar duct diameter ratio (Dout:Din) was significantly lower 3 days after pneumonectomy compared to all controls except for the detergent-treated lung (P < 0.001). To identify clumped capillaries predicted by septal retraction, vascular casting, analyzed by both scanning electron microscopy and synchrotron imaging, demonstrated matted capillaries that were most prominent 3 days after pneumonectomy. Numerical simulations suggested that septal retraction could reflect increased surface tension within the alveolar duct, resulting in a new equilibrium at a higher total energy and lower surface area. The spatial and temporal association of these microstructural changes with postpneumonectomy lung growth suggests that these changes represent an early phase of alveolar duct remodeling.
Keywords: electron microscopy; microstructure; regeneration; surface tension.
Copyright © 2015 the American Physiological Society.
Figures



References
-
- Bachofen H, Gehr P, Weibel ER. Alterations of mechanical-properties and morphology in excised rabbit lungs rinsed with a detergent. J Appl Physiol 47: 1002–1010, 1979. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

