Kinetic-dependent Killing of Oral Pathogens with Nitric Oxide
- PMID: 26078424
- PMCID: PMC4530389
- DOI: 10.1177/0022034515589314
Kinetic-dependent Killing of Oral Pathogens with Nitric Oxide
Abstract
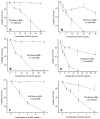
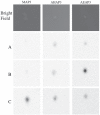
Nitric oxide (NO)-releasing silica nanoparticles were synthesized via the co-condensation of tetramethyl orthosilicate with aminosilanes and subsequent conversion of secondary amines to N-diazeniumdiolate NO donors. A series of ~150 nm NO-releasing particles with different NO totals and release kinetics (i.e., half-lives) were achieved by altering both the identity and mol% composition of the aminosilane precursors. Independent of identical 2 h NO-release totals, enhanced antibacterial action was observed against the periodontopathogens Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis with extended NO-release kinetics at pH 7.4. Negligible bactericidal effect was observed against cariogenic Streptococcus mutans at pH 7.4, even when using NO-releasing silica particles with greater NO-release totals. However, antibacterial activity was observed against S. mutans at lower pH (6.4). This result was attributed to more rapid proton-initiated decomposition of the N-diazeniumdiolate NO donors and greater NO-release payloads. The data suggest a differential sensitivity to NO between cariogenic and periodontopathogenic bacteria with implications for the future development of NO-releasing oral care therapeutics.
Keywords: Aggregatibacter actinomycetemcomitans; N-diazeniumdiolate; Porphyromonas gingivalis; dental caries; periodontal disease; silica.
© International & American Associations for Dental Research 2015.
Conflict of interest statement
The corresponding author declares a competing financial interest. Mark Schoenfisch is a co-founder, is a member of the board of directors, and maintains a financial interest in Novan Therapeutics, Inc. Novan is commercializing macromolecular nitric oxide storage and release vehicles for dermatological indications. The authors declare no further potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures


References
-
- Allaker R, Silva Mendez L, Hardie J, Benjamin N. 2001. Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol Immunol. 16(4):253–256. - PubMed
-
- Aoki W, Kuroda K, Ueda M. 2012. Next generation of antimicrobial peptides as molecular targeted medicines. J Biosci Bioeng. 114(4):365–370. - PubMed
-
- Barbe C, Bartlett J, Kong L, Finnie K, Lin HQ, Larkin M, Calleja S, Bush A, Calleja G. 2004. Silica particles: a novel drug-delivery system. Adv Mater. 16(21):1959–1966.
-
- Bogdan C. 2001. Nitric oxide and the immune response. Nat Immunol. 2(10):907–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

