Transforming Growth Factor-Beta and Oxidative Stress Interplay: Implications in Tumorigenesis and Cancer Progression
- PMID: 26078812
- PMCID: PMC4452864
- DOI: 10.1155/2015/654594
Transforming Growth Factor-Beta and Oxidative Stress Interplay: Implications in Tumorigenesis and Cancer Progression
Abstract
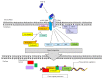
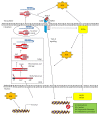
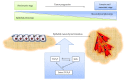
Transforming growth factor-beta (TGF-β) and oxidative stress/Reactive Oxygen Species (ROS) both have pivotal roles in health and disease. In this review we are analyzing the interplay between TGF-β and ROS in tumorigenesis and cancer progression. They have contradictory roles in cancer progression since both can have antitumor effects, through the induction of cell death, senescence and cell cycle arrest, and protumor effects by contributing to cancer cell spreading, proliferation, survival, and metastasis. TGF-β can control ROS production directly or by downregulating antioxidative systems. Meanwhile, ROS can influence TGF-β signaling and increase its expression as well as its activation from the latent complex. This way, both are building a strong interplay which can be taken as an advantage by cancer cells in order to increment their malignancy. In addition, both TGF-β and ROS are able to induce cell senescence, which in one way protects damaged cells from neoplastic transformation but also may collaborate in cancer progression. The mutual collaboration of TGF-β and ROS in tumorigenesis is highly complex, and, due to their differential roles in tumor progression, careful consideration should be taken when thinking of combinatorial targeting in cancer therapies.
Figures
References
-
- Caulin C., Scholl F. G., Frontelo P., Gamallo C., Quintanilla M. Chronic exposure of cultured transformed mouse epidermal cells to transforming growth factor-β1 induces an epithelial-mesenchymal transdifferentiation and a spindle tumoral phenotype. Cell Growth and Differentiation. 1995;6(8):1027–1035. - PubMed
-
- Wikström P., Stattin P., Franck-Lissbrant I., Damber J.-E., Bergh A. Transforming growth factor β1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate. 1998;37(1):19–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

