Oxidative Stress and Protein Quality Control Systems in the Aged Canine Brain as a Model for Human Neurodegenerative Disorders
- PMID: 26078824
- PMCID: PMC4442305
- DOI: 10.1155/2015/940131
Oxidative Stress and Protein Quality Control Systems in the Aged Canine Brain as a Model for Human Neurodegenerative Disorders
Abstract
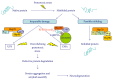
Aged dogs are considered the most suitable spontaneous animal model for studying normal aging and neurodegenerative diseases. Elderly canines naturally develop cognitive dysfunction and neuropathological hallmarks similar to those seen in humans, especially Alzheimer's disease-like pathology. Pet dogs also share similar living conditions and diets to humans. Oxidative damage accumulates in the canine brain during aging, making dogs a valid model for translational antioxidant treatment/prevention studies. Evidence suggests the presence of detective protein quality control systems, involving ubiquitin-proteasome system (UPS) and Heat Shock Proteins (HSPs), in the aged canine brain. Further studies on the canine model are needed to clarify the role of age-related changes in UPS activity and HSP expression in neurodegeneration in order to design novel treatment strategies, such as HSP-based therapies, aimed at improving chaperone defences against proteotoxic stress affecting brain during aging.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

