Stereocontrolled 1,2- cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry
- PMID: 26078847
- PMCID: PMC4465199
- DOI: 10.1039/C5SC00280J
Stereocontrolled 1,2- cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry
Abstract
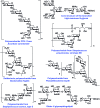
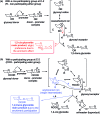
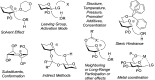
Recent developments in stereoselective 1,2-cis glycosylation that have emerged during the past decade are surveyed herein. For detailed coverage of the previous achievements in the field the reader is referred to our earlier reviews: A. V. Demchenko, Curr. Org. Chem., 2003, 7, 35–79 and Synlett, 2003, 1225–1240.
Figures

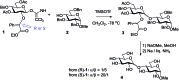
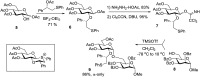

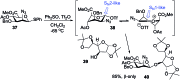

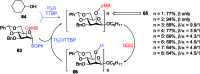





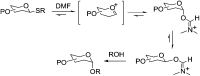






References
-
- Varki A., Cummings R. D., Esko J. D., Freeze H. H., Bertozzi C. R., Stanley P., Hart G. W. and Etzler M. E., Essentials of Glycobiology, CSH Laboratory Press, New York, 2nd edn, 2009. - PubMed
-
- Cao H., Hwang J. and Chen X., in Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry, ed. V. K. Tiwari and B. B. Mishra, 2011, pp. 411–431.
-
- Zhao C., Li M., Luo Y., Wu W. Carbohydr. Res. 2006;341:485–491. - PubMed
-
- Bittencourt V. C. B., Figueiredo R. T., da Silva R. B., Mourão-Sá D. S., Fernandez P. L., Sassaki G. L., Mulloy B., Bozza M. T., Barreto-Bergter E. J. Biol. Chem. 2006;281:22614–22623. - PubMed
- Lopes L. C. L., da Silva M. I. D., Bittencourt V. C. B., Figueiredo R. T., Rollin-Pinheiro R., Sassaki G. L., Bozza M. T., Gorin P. A. J., Barreto-Bergter E. Mycoses. 2011;54:28–36. - PubMed
-
- Tzianabos A. O., Pantosti A., Baumann H., Brisson J. R., Jennings H. J., Kasper D. L. J. Biol. Chem. 1992;267:18230–18235. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

