Achalasia--An Autoimmune Inflammatory Disease: A Cross-Sectional Study
- PMID: 26078981
- PMCID: PMC4452860
- DOI: 10.1155/2015/729217
Achalasia--An Autoimmune Inflammatory Disease: A Cross-Sectional Study
Abstract
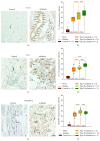
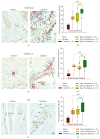
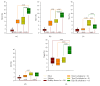
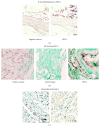
Idiopathic achalasia is a disease of unknown etiology. The loss of myenteric plexus associated with inflammatory infiltrates and autoantibodies support the hypothesis of an autoimmune mechanism. Thirty-two patients diagnosed by high-resolution manometry with achalasia were included. Twenty-six specimens from lower esophageal sphincter muscle were compared with 5 esophagectomy biopsies (control). Immunohistochemical (biopsies) and flow cytometry (peripheral blood) analyses were performed. Circulating anti-myenteric autoantibodies were evaluated by indirect immunofluorescence. Herpes simplex virus-1 (HSV-1) infection was determined by in situ hybridization, RT-PCR, and immunohistochemistry. Histopathological analysis showed capillaritis (51%), plexitis (23%), nerve hypertrophy (16%), venulitis (7%), and fibrosis (3%). Achalasia tissue exhibited an increase in the expression of proteins involved in extracellular matrix turnover, apoptosis, proinflammatory and profibrogenic cytokines, and Tregs and Bregs versus controls (P < 0.001). Circulating Th22/Th17/Th2/Th1 percentage showed a significant increase versus healthy donors (P < 0.01). Type III achalasia patients exhibited the highest inflammatory response versus types I and II. Prevalence of both anti-myenteric antibodies and HSV-1 infection in achalasia patients was 100% versus 0% in controls. Our results suggest that achalasia is a disease with an important local and systemic inflammatory autoimmune component, associated with the presence of specific anti-myenteric autoantibodies, as well as HSV-1 infection.
Figures




References
-
- Mayberry J. F. Epidemiology and demographics of achalasia. Gastrointestinal Endoscopy Clinics of North America. 2001;11(2):235–247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

