Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study
- PMID: 26079503
- PMCID: PMC4469461
- DOI: 10.1371/journal.pmed.1001841
Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study
Abstract
Background: Potentially modifiable risk factors including obesity, diabetes, hypertension, and smoking are associated with Alzheimer disease (AD) and represent promising targets for intervention. However, the causality of these associations is unclear. We sought to assess the causal nature of these associations using Mendelian randomization (MR).
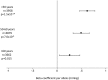
Methods and findings: We used SNPs associated with each risk factor as instrumental variables in MR analyses. We considered type 2 diabetes (T2D, NSNPs = 49), fasting glucose (NSNPs = 36), insulin resistance (NSNPs = 10), body mass index (BMI, NSNPs = 32), total cholesterol (NSNPs = 73), HDL-cholesterol (NSNPs = 71), LDL-cholesterol (NSNPs = 57), triglycerides (NSNPs = 39), systolic blood pressure (SBP, NSNPs = 24), smoking initiation (NSNPs = 1), smoking quantity (NSNPs = 3), university completion (NSNPs = 2), and years of education (NSNPs = 1). We calculated MR estimates of associations between each exposure and AD risk using an inverse-variance weighted approach, with summary statistics of SNP-AD associations from the International Genomics of Alzheimer's Project, comprising a total of 17,008 individuals with AD and 37,154 cognitively normal elderly controls. We found that genetically predicted higher SBP was associated with lower AD risk (odds ratio [OR] per standard deviation [15.4 mm Hg] of SBP [95% CI]: 0.75 [0.62-0.91]; p = 3.4 × 10(-3)). Genetically predicted higher SBP was also associated with a higher probability of taking antihypertensive medication (p = 6.7 × 10(-8)). Genetically predicted smoking quantity was associated with lower AD risk (OR per ten cigarettes per day [95% CI]: 0.67 [0.51-0.89]; p = 6.5 × 10(-3)), although we were unable to stratify by smoking history; genetically predicted smoking initiation was not associated with AD risk (OR = 0.70 [0.37, 1.33]; p = 0.28). We saw no evidence of causal associations between glycemic traits, T2D, BMI, or educational attainment and risk of AD (all p > 0.1). Potential limitations of this study include the small proportion of intermediate trait variance explained by genetic variants and other implicit limitations of MR analyses.
Conclusions: Inherited lifetime exposure to higher SBP is associated with lower AD risk. These findings suggest that higher blood pressure--or some environmental exposure associated with higher blood pressure, such as use of antihypertensive medications--may reduce AD risk.
Conflict of interest statement
SDØ has received speaking fees, consultant honoraria and travel support from Janssen-Cilag until April 2011. Furthermore, he has received travel support at one occasion in 2010 from Bristol-Myers Squibb. EBL reports grants from National Institute on Aging during the conduct of the study, and personal fees from UpToDate outside the submitted work. All other authors have nothing to disclose.
Figures



References
Publication types
MeSH terms
Grants and funding
- P50 AG008671/AG/NIA NIH HHS/United States
- AG05144/AG/NIA NIH HHS/United States
- MC_G1000734/MRC_/Medical Research Council/United Kingdom
- P30 AG013854/AG/NIA NIH HHS/United States
- P50 MH060451/MH/NIMH NIH HHS/United States
- K01 AG030514/AG/NIA NIH HHS/United States
- MH60451/MH/NIMH NIH HHS/United States
- UL1 TR001445/TR/NCATS NIH HHS/United States
- R01 AG022374/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- R01 AG17917/AG/NIA NIH HHS/United States
- U01 HG006375/HG/NHGRI NIH HHS/United States
- M01 RR000096/RR/NCRR NIH HHS/United States
- P30 AG028377/AG/NIA NIH HHS/United States
- RC2 AG036528/AG/NIA NIH HHS/United States
- AG041232/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- U01 AG10483/AG/NIA NIH HHS/United States
- P30 AG10133/AG/NIA NIH HHS/United States
- R01 AG035137/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P50 AG005128/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- R01 AG031581/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- MR/L501529/1/MRC_/Medical Research Council/United Kingdom
- P50 AG016577/AG/NIA NIH HHS/United States
- MC_U123160651/MRC_/Medical Research Council/United Kingdom
- R01 AG019085/AG/NIA NIH HHS/United States
- U01 AG032984/AG/NIA NIH HHS/United States
- U01 HG008657/HG/NHGRI NIH HHS/United States
- 167/ALZS_/Alzheimer's Society/United Kingdom
- R01 AG042611/AG/NIA NIH HHS/United States
- R01 AG013616/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- R01 AG030146/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- UL1 RR029893/RR/NCRR NIH HHS/United States
- P50 AG016575/AG/NIA NIH HHS/United States
- CAPMC/ CIHR/Canada
- HHMI/Howard Hughes Medical Institute/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P50 NS039764/NS/NINDS NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- G0300429/MRC_/Medical Research Council/United Kingdom
- AG05128/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- RC2 AG036502/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- 1R01AG035137/AG/NIA NIH HHS/United States
- R01 MH080295/MH/NIMH NIH HHS/United States
- P01 AG03991/AG/NIA NIH HHS/United States
- R01 AG026390/AG/NIA NIH HHS/United States
- U01 AG 06781/AG/NIA NIH HHS/United States
- G0902227/MRC_/Medical Research Council/United Kingdom
- RC2 AG036535/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- AG010491/AG/NIA NIH HHS/United States
- P30 AG08051/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- NS39764/NS/NINDS NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- 089703/WT_/Wellcome Trust/United Kingdom
- MC_UU_12015/1/MRC_/Medical Research Council/United Kingdom
- AG041718/AG/NIA NIH HHS/United States
- R01 AG012101/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- MC_U106179471/MRC_/Medical Research Council/United Kingdom
- 5R01AG022374/AG/NIA NIH HHS/United States
- P50 AG016570/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- MC_UU_12015/2/MRC_/Medical Research Council/United Kingdom
- P01 AG019724/AG/NIA NIH HHS/United States
- R01AG33193/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- U54 CA132378/CA/NCI NIH HHS/United States
- MC_U106179472/MRC_/Medical Research Council/United Kingdom
- R01 AG033193/AG/NIA NIH HHS/United States
- 1RC2AG036502/AG/NIA NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- AG030653/AG/NIA NIH HHS/United States
- R01 AG15819/AG/NIA NIH HHS/United States
- U24 AG026390/AG/NIA NIH HHS/United States
- AG025688/AG/NIA NIH HHS/United States
- MR/L023784/1/MRC_/Medical Research Council/United Kingdom
- R37 AG015473/AG/NIA NIH HHS/United States
- U24 AG026395/AG/NIA NIH HHS/United States
- AG027944/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- R01 CA129769/CA/NCI NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- U01 AG010483/AG/NIA NIH HHS/United States
- P01 AG002219/AG/NIA NIH HHS/United States
- U01 AG006781/AG/NIA NIH HHS/United States
- U01 AG06781/AG/NIA NIH HHS/United States
- P50 AG005144/AG/NIA NIH HHS/United States
- P01 AG010491/AG/NIA NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- R01 AG021547/AG/NIA NIH HHS/United States
- R01 AG041232/AG/NIA NIH HHS/United States
- R01 AG019757/AG/NIA NIH HHS/United States
- R01 AG020688/AG/NIA NIH HHS/United States
- MR/K006584/1/MRC_/Medical Research Council/United Kingdom
- 5R01AG012101/AG/NIA NIH HHS/United States
- R01 AG030653/AG/NIA NIH HHS/United States
- R01 AG027944/AG/NIA NIH HHS/United States
- R01 AG30146/AG/NIA NIH HHS/United States
- R01 AG017173/AG/NIA NIH HHS/United States
- R01 AG025259/AG/NIA NIH HHS/United States
- U01 HG004610/HG/NHGRI NIH HHS/United States
- R01 AG011101/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P50 AG016582/AG/NIA NIH HHS/United States
- R01 AG041718/AG/NIA NIH HHS/United States
- 5R01AG013616/AG/NIA NIH HHS/United States
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
- MR/L010305/1/MRC_/Medical Research Council/United Kingdom
- P50 AG016576/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- AG019757/AG/NIA NIH HHS/United States
- MO1RR00096/RR/NCRR NIH HHS/United States
- R01 AG026916/AG/NIA NIH HHS/United States
- 081864/WT_/Wellcome Trust/United Kingdom
- G0400713/MRC_/Medical Research Council/United Kingdom
- R01 NS059873/NS/NINDS NIH HHS/United States
- AG021547/AG/NIA NIH HHS/United States
- UL1RR02777/RR/NCRR NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

