AF1q is a novel TCF7 co-factor which activates CD44 and promotes breast cancer metastasis
- PMID: 26079538
- PMCID: PMC4653036
- DOI: 10.18632/oncotarget.4136
AF1q is a novel TCF7 co-factor which activates CD44 and promotes breast cancer metastasis
Abstract
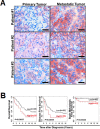
AF1q is an MLL fusion partner that was identified from acute myeloid leukemia (AML) patients with t (1; 11) (q21; q23) chromosomal abnormality. The function of AF1q is not yet fully known, however, elevated AF1q expression is associated with poor clinical outcomes in various malignancies. Here, we show that AF1q specifically binds to T-cell-factor-7 (TCF7) in the Wnt signaling pathway and results in transcriptional activation of CD44 as well as multiple downstream targets of the TCF7/LEF1. In addition, enhanced AF1q expression promotes breast cancer cell proliferation, migration, mammosphere formation, and chemo-resistance. In xenograft models, enforced AF1q expression in breast cancer cells also promotes liver metastasis and lung colonization. In a cohort of 63 breast cancer patients, higher percentages of AF1q-positive cancer cells in primary sites were associated with significantly poorer overall survival (OS), disease-free survival (DFS), and brain metastasis-free survival (b-MFS). Using paired primary/metastatic samples from the same patients, we demonstrate that AF1q-positive breast cancer cells become dynamically dominant in the metastatic sites compared to the primary sites. Our findings indicate that breast cancer cells with a hyperactive AF1q/TCF7/CD44 regulatory axis in the primary sites may represent "metastatic founder cells" which have invasive properties.
Keywords: AF1q; CD44; TCF7; Wnt; metastasis.
Conflict of interest statement
The authors disclose no potential conflict of interest.
Figures





References
-
- Tse W, Zhu W, Chen HS, Cohen A. A novel gene, AF1q, fused to MLL in t(1;11) (q21;q23), is specifically expressed in leukemic and immature hematopoietic cells. Blood. 1995;85:650–656. - PubMed
-
- Xiong Y, Li Z, Ji M, Tan AC, Bemis J, Tse JV, Huang G, Park J, Ji C, Chen J, Bemis LT, Bunting KD, Tse W. MIR29B regulates expression of MLLT11 (AF1Q), an MLL fusion partner, and low MIR29B expression associates with adverse cytogenetics and poor overall survival in AML. British journal of haematology. 2011;153:753–757. - PMC - PubMed
-
- Co NN, Tsang WP, Tsang TY, Yeung CL, Yau PL, Kong SK, Kwok TT. AF1q enhancement of gamma irradiation-induced apoptosis by up-regulation of BAD expression via NF-kappaB in human squamous carcinoma A431 cells. Oncology reports. 2010;24:547–554. - PubMed
-
- Jacques C, Baris O, Prunier-Mirebeau D, Savagner F, Rodien P, Rohmer V, Franc B, Guyetant S, Malthiery Y, Reynier P. Two-step differential expression analysis reveals a new set of genes involved in thyroid oncocytic tumors. The Journal of clinical endocrinology and metabolism. 2005;90:2314–2320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P 26011/FWF_/Austrian Science Fund FWF/Austria
- 5P20RR016440/RR/NCRR NIH HHS/United States
- P20 RR016440/RR/NCRR NIH HHS/United States
- R01CA148671/CA/NCI NIH HHS/United States
- GM103434/GM/NIGMS NIH HHS/United States
- R01 CA148671/CA/NCI NIH HHS/United States
- S10RR026378/RR/NCRR NIH HHS/United States
- U54 GM104942/GM/NIGMS NIH HHS/United States
- GM103488/RR032138/GM/NIGMS NIH HHS/United States
- P30 RR032138/RR/NCRR NIH HHS/United States
- R01 DK059380/DK/NIDDK NIH HHS/United States
- OD016165/OD/NIH HHS/United States
- S10 RR026378/RR/NCRR NIH HHS/United States
- S10 RR020866/RR/NCRR NIH HHS/United States
- P20 GM103434/GM/NIGMS NIH HHS/United States
- P30 GM103488/GM/NIGMS NIH HHS/United States
- P30 RR032138/GM103488/GM/NIGMS NIH HHS/United States
- RR020866/RR/NCRR NIH HHS/United States
- S10 OD016165/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

