Self-repairing symmetry in jellyfish through mechanically driven reorganization
- PMID: 26080418
- PMCID: PMC4491739
- DOI: 10.1073/pnas.1502497112
Self-repairing symmetry in jellyfish through mechanically driven reorganization
Abstract
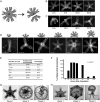
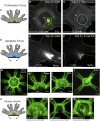
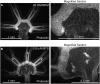
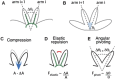
What happens when an animal is injured and loses important structures? Some animals simply heal the wound, whereas others are able to regenerate lost parts. In this study, we report a previously unidentified strategy of self-repair, where moon jellyfish respond to injuries by reorganizing existing parts, and rebuilding essential body symmetry, without regenerating what is lost. Specifically, in response to arm amputation, the young jellyfish of Aurelia aurita rearrange their remaining arms, recenter their manubria, and rebuild their muscular networks, all completed within 12 hours to 4 days. We call this process symmetrization. We find that symmetrization is not driven by external cues, cell proliferation, cell death, and proceeded even when foreign arms were grafted on. Instead, we find that forces generated by the muscular network are essential. Inhibiting pulsation using muscle relaxants completely, and reversibly, blocked symmetrization. Furthermore, we observed that decreasing pulse frequency using muscle relaxants slowed symmetrization, whereas increasing pulse frequency by lowering the magnesium concentration in seawater accelerated symmetrization. A mathematical model that describes the compressive forces from the muscle contraction, within the context of the elastic response from the mesoglea and the ephyra geometry, can recapitulate the recovery of global symmetry. Thus, self-repair in Aurelia proceeds through the reorganization of existing parts, and is driven by forces generated by its own propulsion machinery. We find evidence for symmetrization across species of jellyfish (Chrysaora pacifica, Mastigias sp., and Cotylorhiza tuberculata).
Keywords: jellyfish; propulsion; reorganization; self-repair; symmetry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Graham WM, Kroutil RM. Size-based prey selectivity and dietary shifts in the jellyfish, Aurelia aurita. J Plankton Res. 2001;23(1):67–74.
-
- Purcell JEC, Graham WMC, Dumont HJC. 2001. Jellyfish Blooms: Ecological and Societal Importance. Proceedings of the International Conference on Jellyfish Blooms, held in Gulf Shores, Alabama, 12–14 January, 2000. Hydrobiologia (Springer Science and Business Media, Dordrecht, The Netherlands), Vol 451, p 2001.
-
- Olesen NJ, Riisgård HU. Population dynamic, growth and energetics of jellyfish, Aurelia aurita, in a shallow fjord. Mar Ecol Prog Ser. 1994;105:9–18.
-
- Lucas CH. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia. 2001;451(1-3):229–246.
-
- Conley K, Uye SI. Effects of hyposalinity on survival and settlement of moon jellyfish (Aurelia aurita) planulae. J Exp Mar Biol Ecol. 2015;462:14–19.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

