Three cyanobacteriochromes work together to form a light color-sensitive input system for c-di-GMP signaling of cell aggregation
- PMID: 26080423
- PMCID: PMC4491779
- DOI: 10.1073/pnas.1504228112
Three cyanobacteriochromes work together to form a light color-sensitive input system for c-di-GMP signaling of cell aggregation
Abstract
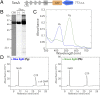
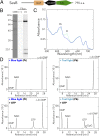
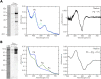
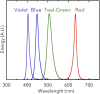
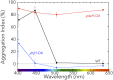
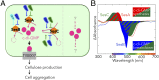
Cyanobacteriochromes (CBCRs) are cyanobacterial photoreceptors that have diverse spectral properties and domain compositions. Although large numbers of CBCR genes exist in cyanobacterial genomes, no studies have assessed whether multiple CBCRs work together. We recently showed that the diguanylate cyclase (DGC) activity of the CBCR SesA from Thermosynechococcus elongatus is activated by blue-light irradiation and that, when irradiated, SesA, via its product cyclic dimeric GMP (c-di-GMP), induces aggregation of Thermosynechococcus vulcanus cells at a temperature that is suboptimum for single-cell viability. For this report, we first characterize the photobiochemical properties of two additional CBCRs, SesB and SesC. Blue/teal light-responsive SesB has only c-di-GMP phosphodiesterase (PDE) activity, which is up-regulated by teal light and GTP. Blue/green light-responsive SesC has DGC and PDE activities. Its DGC activity is enhanced by blue light, whereas its PDE activity is enhanced by green light. A ΔsesB mutant cannot suppress cell aggregation under teal-green light. A ΔsesC mutant shows a less sensitive cell-aggregation response to ambient light. ΔsesA/ΔsesB/ΔsesC shows partial cell aggregation, which is accompanied by the loss of color dependency, implying that a nonphotoresponsive DGC(s) producing c-di-GMP can also induce the aggregation. The results suggest that SesB enhances the light color dependency of cell aggregation by degrading c-di-GMP, is particularly effective under teal light, and, therefore, seems to counteract the induction of cell aggregation by SesA. In addition, SesC seems to improve signaling specificity as an auxiliary backup to SesA/SesB activities. The coordinated action of these three CBCRs highlights why so many different CBCRs exist.
Keywords: light sensing; photoreceptors; second messenger; sessility; signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Gan F, et al. Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science. 2014;345(6202):1312–1317. - PubMed
-
- Kehoe DM, Gutu A. Responding to color: The regulation of complementary chromatic adaptation. Annu Rev Plant Biol. 2006;57:127–150. - PubMed
-
- Yoshihara S, Ikeuchi M. Phototactic motility in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Photochem Photobiol Sci. 2004;3(6):512–518. - PubMed
-
- Savakis P, et al. Light-induced alteration of c-di-GMP level controls motility of Synechocystis sp. PCC 6803. Mol Microbiol. 2012;85(2):239–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

