Interstitial pericytes decrease in aged mouse kidneys
- PMID: 26081073
- PMCID: PMC4505164
- DOI: 10.18632/aging.100756
Interstitial pericytes decrease in aged mouse kidneys
Abstract
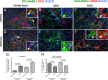
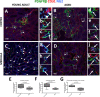
With increasing age, the kidney undergoes characteristic changes in the glomerular and tubulo-interstitial compartments, which are ultimately accompanied by reduced kidney function. Studies have shown age-related loss of peritubular vessels. Normal peritubular vessel tone, function and survival depend on neighboring pericytes. Pericyte detachment leads to vascular damage, which can be accompanied by their differentiation to fibroblasts and myofibroblasts, a state that favors matrix production. To better understand the fate of pericytes in the aged kidney, 27 month-old mice were studied. Compared to 3 month-old young adult mice, aged kidneys showed a substantial decrease in capillaries, identified by CD31 staining, in both cortex and medulla. This was accompanied by a marked decrease in surrounding NG2+ / PDGFRβ+ pericytes. This decrease was more pronounced in the medulla. Capillaries devoid of pericytes were typically dilated in aged mice. Aged kidneys were also characterized by interstitial fibrosis due to increased collagen-I and -III staining. This was accompanied by an increase in the number of pericytes that acquired a pro-fibrotic phenotype, identified by increased PDGFRβ+ / αSMA+ staining. These findings are consistent with the decline in kidney interstitial pericytes as a critical step in the development of changes to the peritubular vasculature with aging, and accompanying fibrosis.
Keywords: NG2; PDGFβ-receptor; endothelium; nephropathy; tubulo-interstitial fibrosis.
Conflict of interest statement
None of the authors have any financial or other conflicts of interest. The results presented in this paper have not been published previously in whole or part.
Figures



References
-
- Brown WW, Abrass IB, Oreopoulos DG. Introduction: aging and the kidney. Advances in renal replacement therapy. 2000;7:1–3. - PubMed
-
- Abrass CK. The nature of chronic progressive nephropathy in aging rats. Advances in renal replacement therapy. 2000;7:4–10. - PubMed
-
- Abdel-Rahman EM, Okusa MD. Effects of aging on renal function and regenerative capacity. Nephron Clinical practice. 2014;127:15–20. - PubMed
-
- Schmitt R, Cantley LG. The impact of aging on kidney repair. American journal of physiology Renal physiology. 2008;294:F1265–1272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

