Autoantigen microarrays reveal autoantibodies associated with proliferative nephritis and active disease in pediatric systemic lupus erythematosus
- PMID: 26081107
- PMCID: PMC4493823
- DOI: 10.1186/s13075-015-0682-6
Autoantigen microarrays reveal autoantibodies associated with proliferative nephritis and active disease in pediatric systemic lupus erythematosus
Abstract
Introduction: Pediatric systemic lupus erythematosus (pSLE) patients often initially present with more active and severe disease than adults, including a higher frequency of lupus nephritis. Specific autoantibodies, including anti-C1q, anti-DNA and anti-alpha-actinin, have been associated with kidney involvement in SLE, and DNA antibodies are capable of initiating early-stage lupus nephritis in severe combined immunodeficiency (SCID) mice. Over 100 different autoantibodies have been described in SLE patients, highlighting the need for comprehensive autoantibody profiling. Knowledge of the antibodies associated with pSLE and proliferative nephritis will increase the understanding of SLE pathogenesis, and may aid in monitoring patients for renal flare.
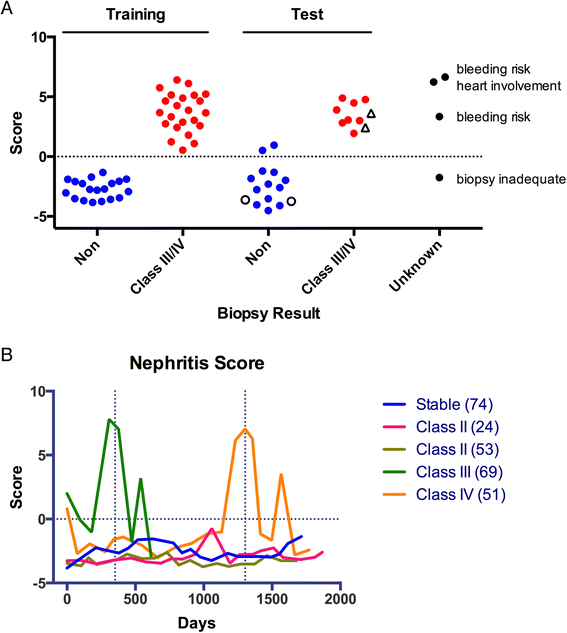
Methods: We used autoantigen microarrays composed of 140 recombinant or purified antigens to compare the serum autoantibody profiles of new-onset pSLE patients (n = 45) to healthy controls (n = 17). We also compared pSLE patients with biopsy-confirmed class III or IV proliferative nephritis (n = 23) and without significant renal involvement (n = 18). We performed ELISA with selected autoantigens to validate the microarray findings. We created a multiple logistic regression model, based on the ELISA and clinical information, to predict whether a patient had proliferative nephritis, and used a validation cohort (n = 23) and longitudinal samples (88 patient visits) to test its accuracy.
Results: Fifty autoantibodies were at significantly higher levels in the sera of pSLE patients compared to healthy controls, including anti-B cell-activating factor (BAFF). High levels of anti-BAFF were associated with active disease. Thirteen serum autoantibodies were present at significantly higher levels in pSLE patients with proliferative nephritis than those without, and we confirmed five autoantigens (dsDNA, C1q, collagens IV and X and aggrecan) by ELISA. Our model, based on ELISA measurements and clinical variables, correctly identified patients with proliferative nephritis with 91 % accuracy.
Conclusions: Autoantigen microarrays are an ideal platform for identifying autoantibodies associated with both pSLE and specific clinical manifestations of pSLE. Using multiple regression analysis to integrate autoantibody and clinical data permits accurate prediction of clinical manifestations with complex etiologies in pSLE.
Figures




References
-
- ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990;33:634–43. doi: 10.1002/art.1780330505. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

