A non-tight junction function of claudin-7-Interaction with integrin signaling in suppressing lung cancer cell proliferation and detachment
- PMID: 26081244
- PMCID: PMC4470020
- DOI: 10.1186/s12943-015-0387-0
A non-tight junction function of claudin-7-Interaction with integrin signaling in suppressing lung cancer cell proliferation and detachment
Abstract
Background: Claudins are a family of tight junction (TJ) membrane proteins involved in a broad spectrum of human diseases including cancer. Claudin-7 is a unique TJ membrane protein in that it has a strong basolateral membrane distribution in epithelial cells and in tissues. Therefore, this study aims to investigate the functional significance of this non-TJ localization of claudin-7 in human lung cancer cells.
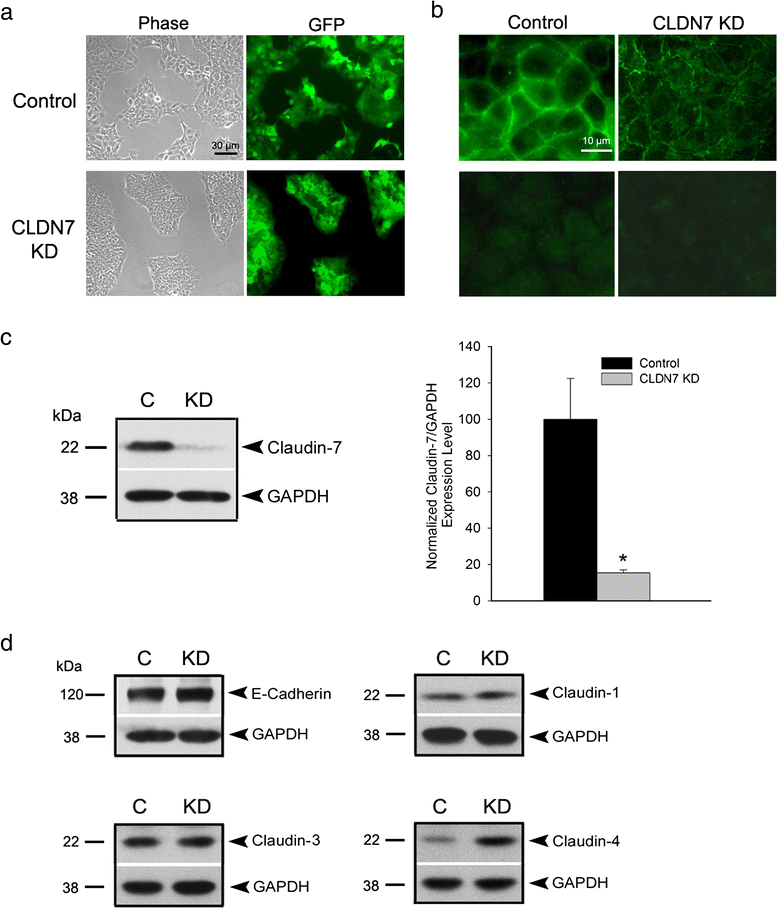
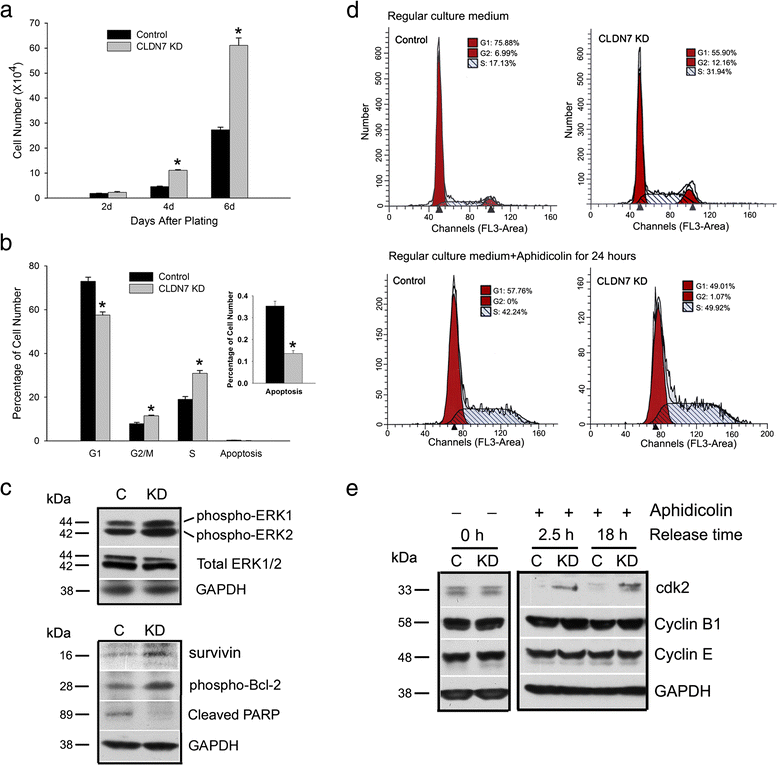
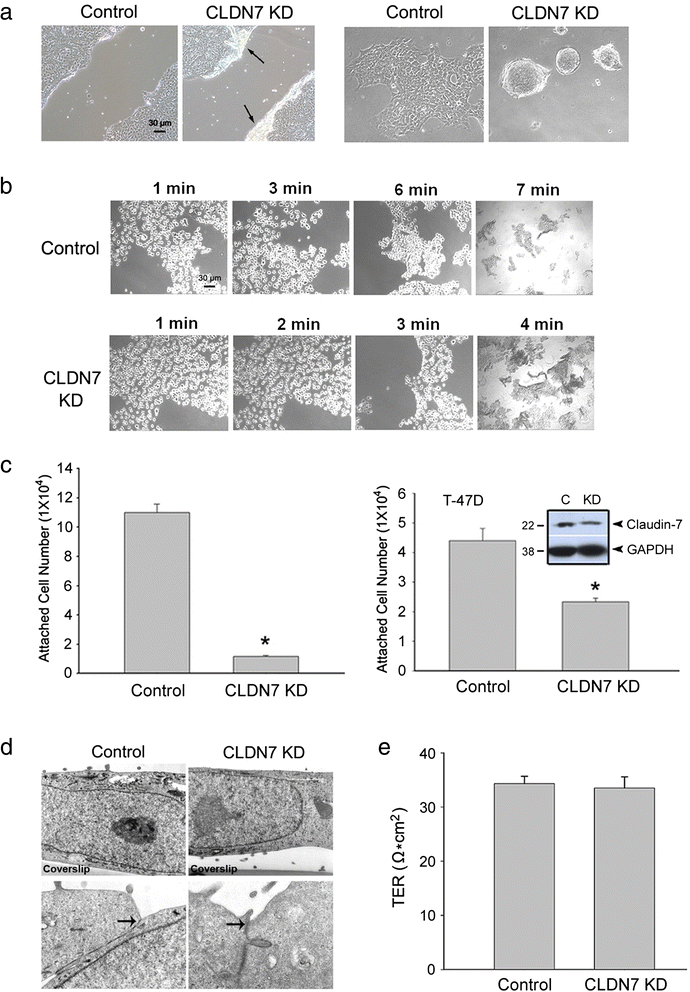
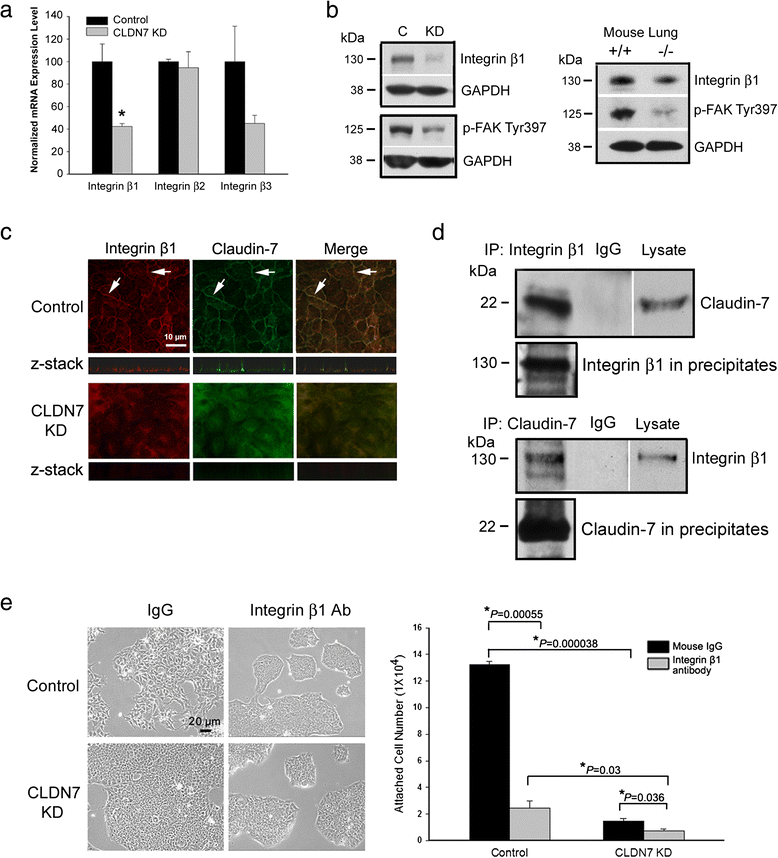
Methods: Claudin-7 expression was suppressed or deleted by lentivirus shRNA or by targeted-gene deletion. Cell cycle analysis and antibody blocking methods were employed to assay cell proliferation and cell attachment, respectively. Electron microscopy and transepthelial electrical resistance measurement were performed to examine the TJ ultrastructure and barrier function. Co-immunolocalization and co-immunoprecipitation was used to study claudin-7 interaction with integrin β1. Tumor growth in vivo were analyzed using athymic nude mice.
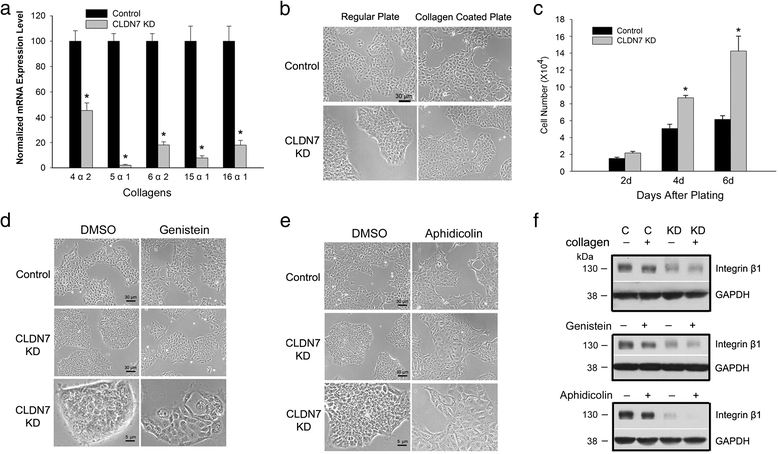
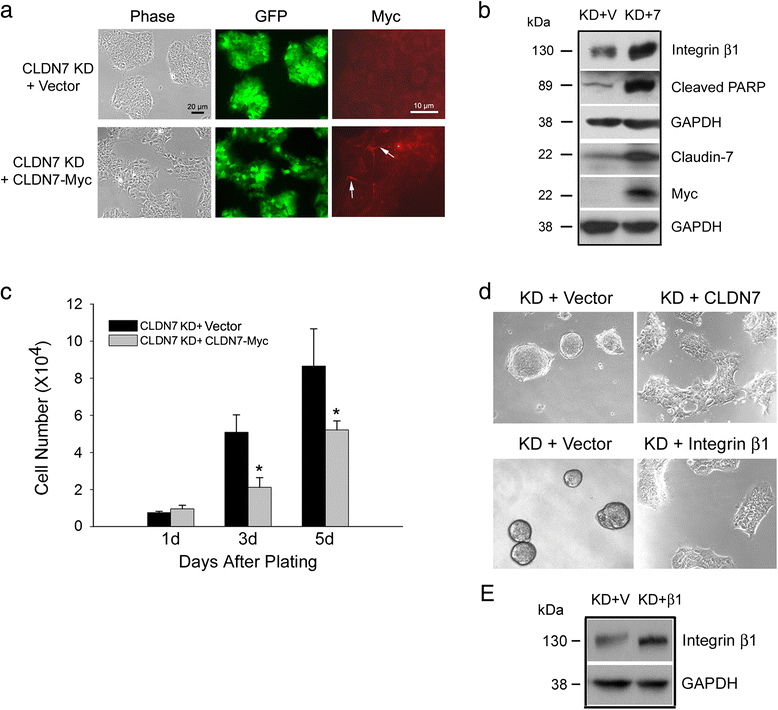
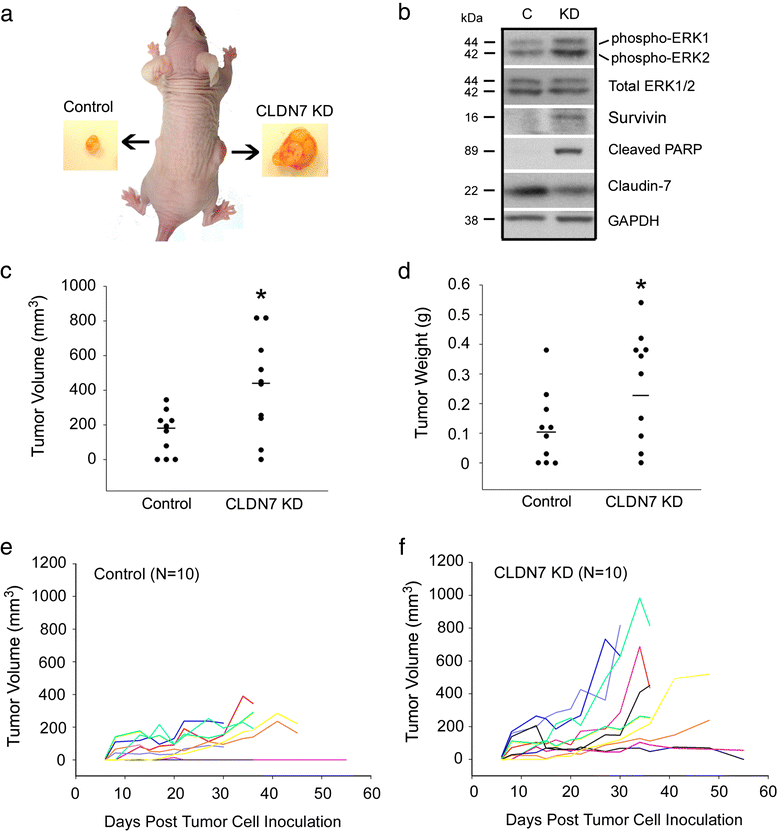
Results: Claudin-7 co-localizes and forms a stable complex with integrin β1. Both suppressing claudin-7 expression by lentivirus shRNA in human lung cancer cells (KD cells) and deletion of claudin-7 in mouse lungs lead to the reduction in integrin β1 and phospho-FAK levels. Suppressing claudin-7 expression increases cell growth and cell cycle progression. More significantly, claudin-7 KD cells have severe defects in cell-matrix interactions and adhere poorly to culture plates with a remarkably reduced integrin β1 expression. When cultured on uncoated glass coverslips, claudin-7 KD cells grow on top of each other and form spheroids while the control cells adhere well and grow as a monolayer. Reintroducing claudin-7 reduces cell proliferation, upregulates integrin β1 expression and increases cell-matrix adhesion. Integrin β1 transfection partially rescues the cell attachment defect. When inoculated into nude mice, claudin-7 KD cells produced significantly larger tumors than control cells.
Conclusion: In this study, we identified a previously unrecognized function of claudin-7 in regulating cell proliferation and maintaining epithelial cell attachment through engaging integrin β1.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

