Orchestrating liver development
- PMID: 26081571
- PMCID: PMC4483763
- DOI: 10.1242/dev.114215
Orchestrating liver development
Abstract
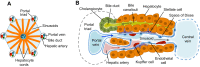
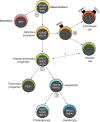
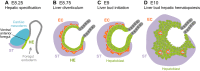
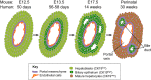
The liver is a central regulator of metabolism, and liver failure thus constitutes a major health burden. Understanding how this complex organ develops during embryogenesis will yield insights into how liver regeneration can be promoted and how functional liver replacement tissue can be engineered. Recent studies of animal models have identified key signaling pathways and complex tissue interactions that progressively generate liver progenitor cells, differentiated lineages and functional tissues. In addition, progress in understanding how these cells interact, and how transcriptional and signaling programs precisely coordinate liver development, has begun to elucidate the molecular mechanisms underlying this complexity. Here, we review the lineage relationships, signaling pathways and transcriptional programs that orchestrate hepatogenesis.
Keywords: Cholangiocyte; Hepatoblast; Hepatocyte; Liver development; Transcription factors.
© 2015. Published by The Company of Biologists Ltd.
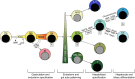
Figures
References
-
- Antoniou A., Raynaud P., Cordi S., Zong Y., Tronche F., Stanger B. Z., Jacquemin P., Pierreux C. E., Clotman F. and Lemaigre F. P. (2009). Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology 136, 2325-2333. 10.1053/j.gastro.2009.02.051 - DOI - PMC - PubMed
-
- Ben-Haim N., Lu C., Guzman-Ayala M., Pescatore L., Mesnard D., Bischofberger M., Naef F., Robertson E. J. and Constam D. B. (2006). The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev. Cell 11, 313-323. 10.1016/j.devcel.2006.07.005 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

