Covalent Modification of Bacteriophage T4 DNA Inhibits CRISPR-Cas9
- PMID: 26081634
- PMCID: PMC4471564
- DOI: 10.1128/mBio.00648-15
Covalent Modification of Bacteriophage T4 DNA Inhibits CRISPR-Cas9
Abstract
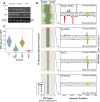
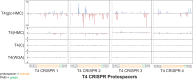
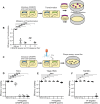
The genomic DNAs of tailed bacteriophages are commonly modified by the attachment of chemical groups. Some forms of DNA modification are known to protect phage DNA from cleavage by restriction enzymes, but others are of unknown function. Recently, the CRISPR-Cas nuclease complexes were shown to mediate bacterial adaptive immunity by RNA-guided target recognition, raising the question of whether phage DNA modifications may also block attack by CRISPR-Cas9. We investigated phage T4 as a model system, where cytosine is replaced with glucosyl-hydroxymethylcytosine (glc-HMC). We first quantified the extent and distribution of covalent modifications in T4 DNA by single-molecule DNA sequencing and enzymatic probing. We then designed CRISPR spacer sequences targeting T4 and found that wild-type T4 containing glc-HMC was insensitive to attack by CRISPR-Cas9 but mutants with unmodified cytosine were sensitive. Phage with HMC showed only intermediate sensitivity. While this work was in progress, another group reported examples of heavily engineered CRISRP-Cas9 complexes that could, in fact, overcome the effects of T4 DNA modification, indicating that modifications can inhibit but do not always fully block attack.
Importance: Bacteria were recently found to have a form of adaptive immunity, the CRISPR-Cas systems, which use nucleic acid pairing to recognize and cleave genomic DNA of invaders such as bacteriophage. Historic work with tailed phages has shown that phage DNA is often modified by covalent attachment of large chemical groups. Here we demonstrate that DNA modification in phage T4 inhibits attack by the CRISPR-Cas9 system. This finding provides insight into mechanisms of host-virus competition and also a new set of tools that may be useful in modulating the activity of CRISPR-Cas9 in genome engineering applications.
Copyright © 2015 Bryson et al.
Figures

References
-
- Karam JD, Drake JW. 1994. Molecular biology of bacteriophage T4. American Society for Microbiology, Washington, DC.
-
- Josse J, Kornberg A. 1962. Glucosylation of deoxyribonucleic acid. III. Alpha- and beta-glucosyl transferases from T4-infected Escherichia coli. J Biol Chem 237:1968–1976. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
