Optogenetic stimulation of GABA neurons can decrease local neuronal activity while increasing cortical blood flow
- PMID: 26082013
- PMCID: PMC4640302
- DOI: 10.1038/jcbfm.2015.140
Optogenetic stimulation of GABA neurons can decrease local neuronal activity while increasing cortical blood flow
Abstract
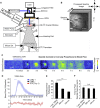
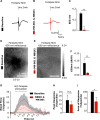
We investigated the link between direct activation of inhibitory neurons, local neuronal activity, and hemodynamics. Direct optogenetic cortical stimulation in the sensorimotor cortex of transgenic mice expressing Channelrhodopsin-2 in GABAergic neurons (VGAT-ChR2) greatly attenuated spontaneous cortical spikes, but was sufficient to increase blood flow as measured with laser speckle contrast imaging. To determine whether the observed optogenetically evoked gamma aminobutyric acid (GABA)-neuron hemodynamic responses were dependent on ionotropic glutamatergic or GABAergic synaptic mechanisms, we paired optogenetic stimulation with application of antagonists to the cortex. Incubation of glutamatergic antagonists directly on the cortex (NBQX and MK-801) blocked cortical sensory evoked responses (as measured with electroencephalography and intrinsic optical signal imaging), but did not significantly attenuate optogenetically evoked hemodynamic responses. Significant light-evoked hemodynamic responses were still present after the addition of picrotoxin (GABA-A receptor antagonist) in the presence of the glutamatergic synaptic blockade. This activation of cortical inhibitory interneurons can mediate large changes in blood flow in a manner that is by and large not dependent on ionotropic glutamatergic or GABAergic synaptic transmission. This supports the hypothesis that activation of inhibitory neurons can increase local cerebral blood flow in a manner that is not entirely dependent on levels of net ongoing neuronal activity.
Figures


References
-
- 1Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci 2006; 29: 449–476. - PubMed
-
- 4Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 2005; 309: 948–951. - PubMed
-
- 5Sumiyoshi A, Suzuki H, Ogawa T, Riera JJ, Shimokawa H, Kawashima R. Coupling between gamma oscillation and fMRI signal in the rat somatosensory cortex: Its dependence on systemic physiological parameters. NeuroImage 2012; 60: 738–746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

