Noninvasive high-speed photoacoustic tomography of cerebral hemodynamics in awake-moving rats
- PMID: 26082016
- PMCID: PMC4527999
- DOI: 10.1038/jcbfm.2015.138
Noninvasive high-speed photoacoustic tomography of cerebral hemodynamics in awake-moving rats
Abstract
We present a noninvasive method of photoacoustic tomography (PAT) for imaging cerebral hemodynamics in awake-moving rats. The wearable PAT (wPAT) system has a size of 15 mm in height and 33 mm in diameter, and a weight of ~8 g (excluding cabling). The wPAT achieved an imaging rate of 3.33 frames/s with a lateral resolution of 243 μm. Animal experiments were designed to show wPAT feasibility for imaging cerebral hemodynamics on awake-moving animals. Results showed that the cerebral oxy-hemoglobin and deoxy-hemoglobin changed significantly in response to hyperoxia; and, after the injection of pentylenetetrazol (PTZ), cerebral blood volume changed faster over time and larger in amplitude for rats in awake-moving state compared with rats under anesthesia. By providing a light-weight, high-resolution technology for in vivo monitoring of cerebral hemodynamics in awake-behaving animals, it will be possible to develop a comprehensive understanding on how activity alters hemodynamics in normal and diseased states.
Figures

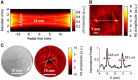
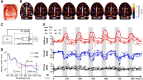

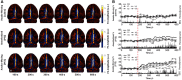

Comment in
-
At long last, PAT hats for the lab rats.J Cereb Blood Flow Metab. 2015 Aug;35(8):1223. doi: 10.1038/jcbfm.2015.136. Epub 2015 Jun 17. J Cereb Blood Flow Metab. 2015. PMID: 26082018 Free PMC article. No abstract available.
References
-
- Kerr JN, Nimmerjahn A. Functional imaging in freely moving animals. Curr Opin Neurobiol. 2012;22:45–53. - PubMed
-
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. - PubMed
-
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

