Analysis of single-cell cytokine secretion reveals a role for paracrine signaling in coordinating macrophage responses to TLR4 stimulation
- PMID: 26082435
- PMCID: PMC5735825
- DOI: 10.1126/scisignal.aaa2155
Analysis of single-cell cytokine secretion reveals a role for paracrine signaling in coordinating macrophage responses to TLR4 stimulation
Abstract
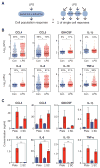
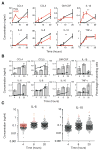
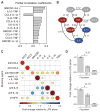
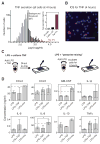
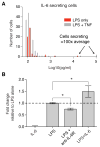
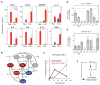
Macrophages not only produce multiple cytokines but also respond to multiple cytokines, which likely shapes the ultimate response of the population. To determine the role of paracrine signaling in shaping the profile of inflammatory cytokines secreted by macrophages in response to stimulation of Toll-like receptor 4 (TLR4) with lipopolysaccharide (LPS), we combined multiplexed, microwell-based measurements of cytokine secretion by single cells with analysis of cytokine secretion by cell populations. Loss of paracrine signaling as a result of cell isolation reduced the secretion by macrophage-like U937 cells and human monocyte-derived macrophages (MDMs) of a subset of LPS-stimulated cytokines, including interleukin-6 (IL-6) and IL-10. Graphical Gaussian modeling (GGM) of the single-cell data defined a regulatory network of paracrine signals, which was validated experimentally in the population through antibody-mediated neutralization of individual cytokines. Tumor necrosis factor-α (TNF-α) was the most influential cytokine in the GGM network. Paracrine signaling by TNF-α secreted from a small subpopulation of "high-secreting" cells was necessary, but not sufficient, for the secretion of large amounts of IL-6 and IL-10 by the cell population. Decreased relative IL-10 secretion by isolated MDMs was linked to increased TNF-α secretion, suggesting that inhibition of the inflammatory response also depends on paracrine signaling. Our results reveal a previously uncharacterized role for cell-to-cell communication within a population in coordinating a rapid innate immune response despite underlying cell-to-cell heterogeneity.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures

References
-
- Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK. The response of human epithelial cells to TNF involves an inducible autocrine cascade. Cell. 2006;124:1225–1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

