Role of Novel Serine 316 Phosphorylation of the p65 Subunit of NF-κB in Differential Gene Regulation
- PMID: 26082493
- PMCID: PMC4536440
- DOI: 10.1074/jbc.M115.639849
Role of Novel Serine 316 Phosphorylation of the p65 Subunit of NF-κB in Differential Gene Regulation
Abstract
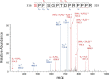
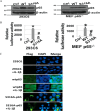
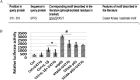
Nuclear factor κB (NF-κB) is a central coordinator in immune and inflammatory responses. Constitutive NF-κB is often found in some types of cancers, contributing to oncogenesis and tumor progression. Therefore, knowing how NF-κB is regulated is important for its therapeutic control. Post-translational modification of the p65 subunit of NF-κB is a well known approach for its regulation. Here, we reported that in response to interleukin 1β, the p65 subunit of NF-κB is phosphorylated on the novel serine 316. Overexpression of S316A (serine 316 → alanine) mutant exhibited significantly reduced ability to activate NF-κB and decreased cell growth as compared with wtp65 (wild type p65). Moreover, conditioned media from cells expressing the S316A-p65 mutant had a considerably lower ability to induce NF-κB than that of wtp65. Our data suggested that phosphorylation of p65 on Ser-316 controls the activity and function of NF-κB. Importantly, we found that phosphorylation at the novel Ser-316 site and other two known phosphorylation sites, Ser-529 and Ser-536, either individually or cooperatively, regulated distinct groups of NF-κB-dependent genes, suggesting the unique role of each individual phosphorylation site on NF-κB-dependent gene regulation. Our novel findings provide an important piece of evidence regarding differential regulation of NF-κB-dependent genes through phosphorylation of different p65 serine residues, thus shedding light on novel mechanisms for the pathway-specific control of NF-κB. This knowledge is key to develop strategies for prevention and treatment of constitutive NF-κB-driven inflammatory diseases and cancers.
Keywords: NF-κB (NF-κB); mass spectrometry (MS); phosphorylation; post-translational modification (PTM); serine.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Gilmore T. D. (2006) Introduction to NF-κB: players, pathways, perspectives. Oncogene 25, 6680–6684 - PubMed
-
- Perkins N. D. (2006) Post-translational modifications regulating the activity and function of the nuclear factor κB pathway. Oncogene 25, 6717–6730 - PubMed
-
- Viatour P., Merville M. P., Bours V., Chariot A. (2005) Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem. Sci. 30, 43–52 - PubMed
-
- Chen L. F., Greene W. C. (2003) Regulation of distinct biological activities of the NF-κB transcription factor complex by acetylation. J. Mol. Med. 81, 549–557 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

