LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD
- PMID: 26082625
- PMCID: PMC4461092
- DOI: 10.2147/COPD.S84436
LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD
Abstract
Background: The current Global initiative for chronic Obstructive Lung Disease (GOLD) treatment strategy recommends the use of one or more bronchodilators according to the patient's airflow limitation, their history of exacerbations, and symptoms. The LANTERN study evaluated the effect of the long-acting β2-agonist (LABA)/long-acting muscarinic antagonist (LAMA) dual bronchodilator, QVA149 (indacaterol/glycopyrronium), as compared with the LABA/inhaled corticosteroid, salmeterol/fluticasone (SFC), in patients with moderate-to-severe COPD with a history of ≤1 exacerbation in the previous year.
Methods: In this double-blind, double-dummy, parallel-group study, 744 patients with moderate-to-severe COPD with a history of ≤1 exacerbations in the previous year were randomized (1:1) to QVA149 110/50 μg once daily or SFC 50/500 μg twice daily for 26 weeks. The primary endpoint was noninferiority of QVA149 versus SFC for trough forced expiratory volume in 1 second (FEV1) at week 26.
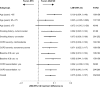
Results: Overall, 676 patients completed the study. The primary objective of noninferiority between QVA149 and SFC in trough FEV1 at week 26 was met. QVA149 demonstrated statistically significant superiority to SFC for trough FEV1 (treatment difference [Δ]=75 mL; P<0.001). QVA149 demonstrated a statistically significant improvement in standardized area under the curve (AUC) from 0 hours to 4 hours for FEV1 (FEV1 AUC0-4h) at week 26 versus SFC (Δ=122 mL; P<0.001). QVA149 and SFC had similar improvements in transition dyspnea index focal score, St George Respiratory Questionnaire total score, and rescue medication use. However, QVA149 significantly reduced the rate of moderate or severe exacerbations by 31% (P=0.048) over SFC. Overall, the incidence of adverse events was comparable between QVA149 (40.1%) and SFC (47.4%). The incidence of pneumonia was threefold lower with QVA149 (0.8%) versus SFC (2.7%).
Conclusion: These findings support the use of the LABA/LAMA, QVA149 as an alternative treatment, over LABA/inhaled corticosteroid, in the management of moderate-to-severe COPD patients (GOLD B and GOLD D) with a history of ≤1 exacerbation in the previous year.
Trial registration: ClinicalTrials.gov NCT01709903.
Keywords: COPD; clinical trial; long-acting muscarinic antagonist; long-acting β2-agonists.
Figures



References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: Updated 2015. Seattle, WA: Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2015. [Accessed March 14, 2015]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Feb18.pdf.
-
- Miravitlles M, Sicras A, Crespo C, et al. Costs of chronic obstructive pulmonary disease in relation to compliance with guidelines: a study in the primary care setting. Ther Adv Respir Dis. 2013;7(3):139–150. - PubMed
-
- Rutten-van Mölken MP, Goossens LM. Cost effectiveness of pharmacological maintenance treatment for chronic obstructive pulmonary disease: a review of the evidence and methodological issues. Pharmacoeconomics. 2012;30(4):271–302. - PubMed
-
- Calverley P, Pauwels R, Vestbo J, et al. TRial of Inhaled STeroids ANd long-acting beta2 agonists study group Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–456. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

