Transgenic Eimeria mitis expressing chicken interleukin 2 stimulated higher cellular immune response in chickens compared with the wild-type parasites
- PMID: 26082759
- PMCID: PMC4451583
- DOI: 10.3389/fmicb.2015.00533
Transgenic Eimeria mitis expressing chicken interleukin 2 stimulated higher cellular immune response in chickens compared with the wild-type parasites
Abstract
Chicken coccidiosis, caused by Eimeria sp., occurs in almost all poultry farms and causes huge economic losses in the poultry industry. Although this disease could be controlled by vaccination, the reduced feed conservation ratio limits the widespread application of anticoccidial vaccines in broilers because some intermediate and/or low immunogenic Eimeria sp. only elicit partial protection. It is of importance to enhance the immunogenicity of these Eimeria sp. by adjuvants for more effective prevention of coccidiosis. Cytokines have remarkable effects on the immunogenicity of antigens. Interleukin 2 (IL-2), for example, significantly stimulates the activation of CD8+ T cells and other immune cells. In this study, we constructed a transgenic Eimeria mitis line (EmiChIL-2) expressing chicken IL-2 (ChIL-2) to investigate the adjuvant effect of ChIL-2 to enhance the immunogenicity of E. mitis against its infection. Stable transfected EmiChIL-2 population was obtained by pyrimethamine selection and verified by PCR, genome walking, western blotting and indirect immunofluorescence assay. Cellular immune response, E. mitis-specific IFN-γ secretion lymphocytes in the peripheral blood mononuclear cells, stimulated by EmiChIL-2 was analyzed by enzyme-linked immunospot assay (ELISPOT). The results showed that EmiChIL-2 stimulated a higher cellular immune response compared with that of the wild-type parasite infection in chickens. Moreover, after the immunization with EmiChIL-2, elevated cellular immune response as well as reduced oocyst output were observed These results indicated that ChIL-2 expressed by Eimeria sp. functions as adjuvant and IL-2 expressing Eimeria parasites are valuable vaccine strains against coccidiosis.
Keywords: ELISPOT; cellular immune response; chicken interleukin 2; reproductive potential; transgenic Eimeria mitis.
Figures



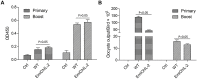

References
-
- Addison C. L., Bramson J. L., Hitt M. M., Muller W. J., Gauldie J., Graham F. L. (1998). Intratumoral coinjection of adenoviral vectors expressing IL-2 and IL-12 results in enhanced frequency of regression of injected and untreated distal tumors. Gene Ther. 5 1400–1409. 10.1038/sj.gt.3300731 - DOI - PubMed
-
- Chapman H. D. (2000). Practical use of vaccines for the control of coccidiosis in the chicken. Worlds Poult. Sci. J. 56 7–20. 10.1079/WPS20000002 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

