Complement System Part I - Molecular Mechanisms of Activation and Regulation
- PMID: 26082779
- PMCID: PMC4451739
- DOI: 10.3389/fimmu.2015.00262
Complement System Part I - Molecular Mechanisms of Activation and Regulation
Abstract
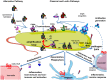
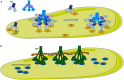
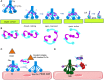
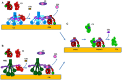
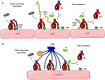
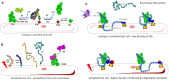
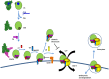
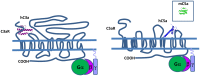
Complement is a complex innate immune surveillance system, playing a key role in defense against pathogens and in host homeostasis. The complement system is initiated by conformational changes in recognition molecular complexes upon sensing danger signals. The subsequent cascade of enzymatic reactions is tightly regulated to assure that complement is activated only at specific locations requiring defense against pathogens, thus avoiding host tissue damage. Here, we discuss the recent advances describing the molecular and structural basis of activation and regulation of the complement pathways and their implication on physiology and pathology. This article will review the mechanisms of activation of alternative, classical, and lectin pathways, the formation of C3 and C5 convertases, the action of anaphylatoxins, and the membrane-attack-complex. We will also discuss the importance of structure-function relationships using the example of atypical hemolytic uremic syndrome. Lastly, we will discuss the development and benefits of therapies using complement inhibitors.
Keywords: alternative complement pathway; anaphylatoxins; classical complement pathway; complement regulatory proteins; complement system proteins; endothelial cells; membrane-attack-complex; structure–function relationships.
Figures







References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

