A Systematic Review and Meta-Analysis on the Safety of Vascular Endothelial Growth Factor (VEGF) Inhibitors for the Treatment of Retinopathy of Prematurity
- PMID: 26083024
- PMCID: PMC4470662
- DOI: 10.1371/journal.pone.0129383
A Systematic Review and Meta-Analysis on the Safety of Vascular Endothelial Growth Factor (VEGF) Inhibitors for the Treatment of Retinopathy of Prematurity
Abstract
Introduction: Laser photocoagulation is the current gold standard treatment for proliferative retinopathy of prematurity (ROP). However, it permanently reduces the visual field and might induce myopia. Vascular endothelial growth factor (VEGF) inhibitors for the treatment of ROP may enable continuing vascularization of the retina, potentially allowing the preservation of the visual field. However, for their use in infants concern remains. This meta-analysis explores the safety of VEGF inhibitors.
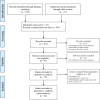
Methods: The Ovid Interface was used to perform a systematic review of the literature in the databases PubMed, EMBASE and the Cochrane Library.
Results: This meta-analysis included 24 original reports (including 1.457 eyes) on VEGF inhibitor treatment for ROP. The trials were solely observational except for one randomized and two case-control studies. We estimated a 6-month risk of retreatment per eye of 2.8%, and a 6-month risk of ocular complication without the need of retreatment of 1.6% per eye. Systemic complications were only reported as isolated incidents.
Discussion: VEGF inhibitors seem to be associated with low recurrence rates and ocular complication rates. They may have the benefit of potentially allowing the preservation of visual field and lower rates of myopia. Due to the lack of data, the risk of systemic side effects cannot be assessed.
Conflict of interest statement
Figures

References
-
- Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, Zin A; International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005. May;115(5):e518–25. Epub 2005 Apr 1. - PubMed
-
- McLoone E, O'Keefe M, McLoone S, Lanigan B. Effect of diode laser retinal ablative therapy for threshold retinopathy of prematurity on the visual field: results of goldmann perimetry at a mean age of 11 years. J Pediatr Ophthalmol Strabismus. 2007. May-Jun;44(3):170–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

