Fucoidan Extracts Ameliorate Acute Colitis
- PMID: 26083103
- PMCID: PMC4471193
- DOI: 10.1371/journal.pone.0128453
Fucoidan Extracts Ameliorate Acute Colitis
Abstract
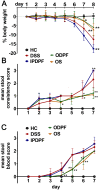
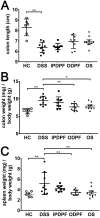
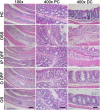
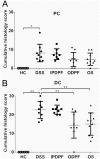
Inflammatory bowel diseases (IBD), such as ulcerative colitis and Crohn's disease, are an important cause of morbidity and impact significantly on quality of life. Overall, current treatments do not sustain a long-term clinical remission and are associated with adverse effects, which highlight the need for new treatment options. Fucoidans are complex sulphated, fucose-rich polysaccharides, found in edible brown algae and are described as having multiple bioactivities including potent anti-inflammatory effects. Therefore, the therapeutic potential of two different fucoidan preparations, fucoidan-polyphenol complex (Maritech Synergy) and depyrogenated fucoidan (DPF) was evaluated in the dextran sulphate sodium (DSS) mouse model of acute colitis. Mice were treated once daily over 7 days with fucoidans via oral (Synergy or DPF) or intraperitoneal administration (DPF). Signs and severity of colitis were monitored daily before colons and spleens were collected for macroscopic evaluation, cytokine measurements and histology. Orally administered Synergy and DPF, but not intraperitoneal DPF treatment, significantly ameliorated symptoms of colitis based on retention of body weight, as well as reduced diarrhoea and faecal blood loss, compared to the untreated colitis group. Colon and spleen weight in mice treated with oral fucoidan was also significantly lower, indicating reduced inflammation and oedema. Histological examination of untreated colitis mice confirmed a massive loss of crypt architecture and goblet cells, infiltration of immune cells and oedema, while all aspects of this pathology were alleviated by oral fucoidan. Importantly, in this model, the macroscopic changes induced by oral fucoidan correlated significantly with substantially decreased production of at least 15 pro-inflammatory cytokines by the colon tissue. Overall, oral fucoidan preparations significantly reduce the inflammatory pathology associated with DSS-induced colitis and could therefore represent a novel nutraceutical option for the management of IBD.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

