A randomized trial of intravenous and oral iron in chronic kidney disease
- PMID: 26083656
- PMCID: PMC4589436
- DOI: 10.1038/ki.2015.163
A randomized trial of intravenous and oral iron in chronic kidney disease
Abstract
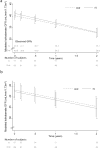
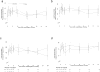
Although iron is commonly used to correct iron deficiency anemia (IDA) in chronic kidney disease (CKD), its effect on kidney function is unclear. To assess this, we randomly assigned patients with stage 3 and 4 CKD and IDA to either open-label oral ferrous sulfate (69 patients to 325 mg three times daily for 8 weeks) or intravenous iron sucrose (67 patients to 200 mg every 2 weeks, total 1 g). The primary outcome was the between-group difference in slope of measured glomerular filtration rate (mGFR) change over two years. The trial was terminated early on the recommendation of an independent data and safety monitoring board based on little chance of finding differences in mGFR slopes, but a higher risk of serious adverse events in the intravenous iron treatment group. mGFR declined similarly over two years in both treatment groups (oral -3.6 ml/min per 1.73 m(2), intravenous -4.0 ml/min per 1.73 m(2), between-group difference -0.35 ml/min per 1.73 m(2); 95% confidence interval -2.9 to 2.3). There were 36 serious cardiovascular events among 19 participants assigned to the oral iron treatment group and 55 events among 17 participants of the intravenous iron group (adjusted incidence rate ratio 2.51 (1.56-4.04)). Infections resulting in hospitalizations had a significant adjusted incidence rate ratio of 2.12 (1.24-3.64). Thus, among non-dialyzed patients with CKD and IDA, intravenous iron therapy is associated with an increased risk of serious adverse events, including those from cardiovascular causes and infectious diseases.
Figures

Comment in
-
Chronic kidney disease: Serious adverse effects associated with IV iron in CKD.Nat Rev Nephrol. 2015 Sep;11(9):506. doi: 10.1038/nrneph.2015.112. Epub 2015 Jul 7. Nat Rev Nephrol. 2015. PMID: 26149838 No abstract available.
-
Oral or intravenous iron for anemia correction in chronic kidney disease?Kidney Int. 2015 Oct;88(4):673-5. doi: 10.1038/ki.2015.189. Kidney Int. 2015. PMID: 26422625
References
-
- Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2001;161:1207–1216. - PubMed
-
- Nissenson AR, Strobos J. Iron deficiency in patients with renal failure. Kidney Int Suppl. 1999;69:S18–S21. - PubMed
-
- Besarab A. Iron and cardiac disease in the end-stage renal disease setting. Am J Kidney Dis. 1999;34:S18–S24. - PubMed
-
- Besarab A, Frinak S, Yee J. An indistinct balance: the safety and efficacy of parenteral iron therapy. J Am Soc Nephrol. 1999;10:2029–2043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

