Competitive binding of antagonistic peptides fine-tunes stomatal patterning
- PMID: 26083750
- PMCID: PMC4532310
- DOI: 10.1038/nature14561
Competitive binding of antagonistic peptides fine-tunes stomatal patterning
Abstract
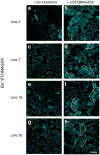
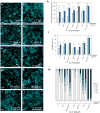
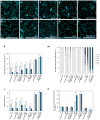
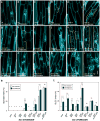
During development, cells interpret complex and often conflicting signals to make optimal decisions. Plant stomata, the cellular interface between a plant and the atmosphere, develop according to positional cues, which include a family of secreted peptides called epidermal patterning factors (EPFs). How these signalling peptides orchestrate pattern formation at a molecular level remains unclear. Here we report in Arabidopsis that Stomagen (also called EPF-LIKE9) peptide, which promotes stomatal development, requires ERECTA (ER)-family receptor kinases and interferes with the inhibition of stomatal development by the EPIDERMAL PATTERNING FACTOR 2 (EPF2)-ER module. Both EPF2 and Stomagen directly bind to ER and its co-receptor TOO MANY MOUTHS. Stomagen peptide competitively replaced EPF2 binding to ER. Furthermore, application of EPF2, but not Stomagen, elicited rapid phosphorylation of downstream signalling components in vivo. Our findings demonstrate how a plant receptor agonist and antagonist define inhibitory and inductive cues to fine-tune tissue patterning on the plant epidermis.
Figures








Comment in
-
Plant science: Precision positioning with peptides.Nature. 2015 Jun 25;522(7557):424-5. doi: 10.1038/nature14535. Epub 2015 Jun 17. Nature. 2015. PMID: 26083745 No abstract available.
References
-
- Fukuda H, Higashiyama T. Diverse functions of plant peptides: entering a new phase. Plant & cell physiology. 2011;52:1–4. - PubMed
-
- Hara K, et al. Epidermal cell density is auto-regulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR2 in Arabidopsis leaves. Plant & cell physiology. 2009;50:1019–1031. - PubMed
-
- Hunt L, Gray JE. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Curr Biol. 2009;19:864–869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

