New cofactor supports α,β-unsaturated acid decarboxylation via 1,3-dipolar cycloaddition
- PMID: 26083754
- PMCID: PMC4988494
- DOI: 10.1038/nature14560
New cofactor supports α,β-unsaturated acid decarboxylation via 1,3-dipolar cycloaddition
Abstract
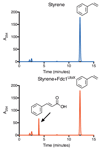
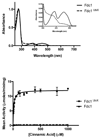
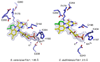
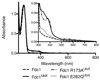
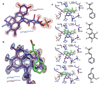
The bacterial ubiD and ubiX or the homologous fungal fdc1 and pad1 genes have been implicated in the non-oxidative reversible decarboxylation of aromatic substrates, and play a pivotal role in bacterial ubiquinone (also known as coenzyme Q) biosynthesis or microbial biodegradation of aromatic compounds, respectively. Despite biochemical studies on individual gene products, the composition and cofactor requirement of the enzyme responsible for in vivo decarboxylase activity remained unclear. Here we show that Fdc1 is solely responsible for the reversible decarboxylase activity, and that it requires a new type of cofactor: a prenylated flavin synthesized by the associated UbiX/Pad1. Atomic resolution crystal structures reveal that two distinct isomers of the oxidized cofactor can be observed, an isoalloxazine N5-iminium adduct and a N5 secondary ketimine species with markedly altered ring structure, both having azomethine ylide character. Substrate binding positions the dipolarophile enoic acid group directly above the azomethine ylide group. The structure of a covalent inhibitor-cofactor adduct suggests that 1,3-dipolar cycloaddition chemistry supports reversible decarboxylation in these enzymes. Although 1,3-dipolar cycloaddition is commonly used in organic chemistry, we propose that this presents the first example, to our knowledge, of an enzymatic 1,3-dipolar cycloaddition reaction. Our model for Fdc1/UbiD catalysis offers new routes in alkene hydrocarbon production or aryl (de)carboxylation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Comment in
-
Biochemistry: Unexpected role for vitamin B2.Nature. 2015 Jun 25;522(7557):427-8. doi: 10.1038/nature14536. Epub 2015 Jun 17. Nature. 2015. PMID: 26083748 No abstract available.
References
-
- Aussel L, Pierrel F, Loiseau L, Lombard M, Fontecave M, Barras F. Biosynthesis and physiology of coenzyme Q in bacteria. Biochim Biophys Acta. 2014;1837:1004–1011. - PubMed
-
- Leppik RA, Young IG, Gibson F. Membrane-associated reactions in ubiquinone biosynthesis in Escherichia coli. 3-octoprenyl-4-hydroxybenzoate carboxy-lyase. Biochim Biophys Acta. 1976;436:800–810. - PubMed
-
- Boll M, Loeffler C, Morris BEL, Kung JW. Anaerobic degradation of homocyclic aromatic compounds via arylcarboxyl-coenzyme A esters: organisms, strategies and key enzymes. Environ Microbiol. 2014;16:612–627. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- BB/M017702/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H021523/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K017802/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M/017702/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/E013007/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

