Exploiting biological and physical determinants of radiotherapy toxicity to individualize treatment
- PMID: 26084351
- PMCID: PMC4628540
- DOI: 10.1259/bjr.20150172
Exploiting biological and physical determinants of radiotherapy toxicity to individualize treatment
Abstract
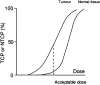
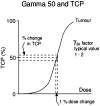
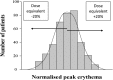
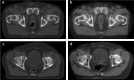
The recent advances in radiation delivery can improve tumour control probability (TCP) and reduce treatment-related toxicity. The use of intensity-modulated radiotherapy (IMRT) in particular can reduce normal tissue toxicity, an objective in its own right, and can allow safe dose escalation in selected cases. Ideally, IMRT should be combined with image guidance to verify the position of the target, since patients, target and organs at risk can move day to day. Daily image guidance scans can be used to identify the position of normal tissue structures and potentially to compute the daily delivered dose. Fundamentally, it is still the tolerance of the normal tissues that limits radiotherapy (RT) dose and therefore tumour control. However, the dose-response relationships for both tumour and normal tissues are relatively steep, meaning that small dose differences can translate into clinically relevant improvements. Differences exist between individuals in the severity of toxicity experienced for a given dose of RT. Some of this difference may be the result of differences between the planned dose and the accumulated dose (DA). However, some may be owing to intrinsic differences in radiosensitivity of the normal tissues between individuals. This field has been developing rapidly, with the demonstration of definite associations between genetic polymorphisms and variation in toxicity recently described. It might be possible to identify more resistant patients who would be suitable for dose escalation, as well as more sensitive patients for whom toxicity could be reduced or avoided. Daily differences in delivered dose have been investigated within the VoxTox research programme, using the rectum as an example organ at risk. In patients with prostate cancer receiving curative RT, considerable daily variation in rectal position and dose can be demonstrated, although the median position matches the planning scan well. Overall, in 10 patients, the mean difference between planned and accumulated rectal equivalent uniform doses was -2.7 Gy (5%), and a dose reduction was seen in 7 of the 10 cases. If dose escalation was performed to take rectal dose back to the planned level, this should increase the mean TCP (as biochemical progression-free survival) by 5%. Combining radiogenomics with individual estimates of DA might identify almost half of patients undergoing radical RT who might benefit from either dose escalation, suggesting improved tumour cure or reduced toxicity or both.
Figures


References
-
- IAEA Human Health Series No. 14. Planning National radiotherapy services: a practical tool. International Atomic Energy Agency, Vienna, 2010.
-
- Kings Fund, London. 2011. [Updated 28 January 2015; cited 5 June 2015]. Available from: http://www.kingsfund.org.uk/sites/files/kf/How-to-improve-cancer-surviva...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

