Maternal Deworming Research Study (MADRES) protocol: a double-blind, placebo-controlled randomised trial to determine the effectiveness of deworming in the immediate postpartum period
- PMID: 26084556
- PMCID: PMC4480032
- DOI: 10.1136/bmjopen-2015-008560
Maternal Deworming Research Study (MADRES) protocol: a double-blind, placebo-controlled randomised trial to determine the effectiveness of deworming in the immediate postpartum period
Abstract
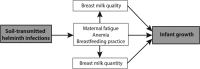
Introduction: Soil-transmitted helminth infections are endemic in 114 countries worldwide, and cause the highest burden of disease among all neglected tropical diseases. The WHO includes women of reproductive age as a high-risk group for infection. The primary consequence of infection in this population is anaemia. During lactation, anaemia may contribute to reduced quality and quantity of milk, decreasing the duration of exclusive breastfeeding and lowering the age at weaning. To date, no study has investigated the effects of maternal postpartum deworming on infant or maternal health outcomes.
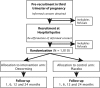
Methods and analysis: A single-centre, parallel, double-blind, randomised, placebo-controlled trial will be carried out in Iquitos, Peru, to assess the effectiveness of integrating single-dose 400 mg albendazole into routine maternal postpartum care. A total of 1010 mother-infant pairs will be randomised to either the intervention or control arm, following inhospital delivery and prior to discharge. Participants will be visited in their homes at 1, 6, 12 and 24 months following delivery for outcome ascertainment. The primary outcome is infant mean weight gain between birth and 6 months of age. Secondary outcomes include other infant growth indicators and morbidity, maternal soil-transmitted helminth infection and intensity, anaemia, fatigue, and breastfeeding practices. All statistical analyses will be performed on an intention-to-treat basis.
Ethics and dissemination: Research ethics board approval has been obtained from the McGill University Health Centre (Canada), the Asociación Civil Impacta Salud y Educación (Peru) and the Instituto Nacional de Salud (Peru). A data safety and monitoring committee is in place to oversee study progression and evaluate adverse events. The results of the analyses will be published in peer-reviewed journals, and presented at national and international conferences.
Trial registration number: Clinicaltrials.gov: NCT01748929.
Keywords: PARASITOLOGY; albendazole; helminthiasis; intervention study; postpartum; randomised controlled trials.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- Hotez PJ, Bundy DAP, Beegle K et al. . Helminth infections: soil-transmitted helminth infections and schistosomiasis. In: Jamison DT, Breman JG, Measham AR et al., eds. Disease control priorities in developing countries. 2 edn New York: Oxford University Press, 2006:467–82.
-
- World Health Organization. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. Geneva: World Health Organization, 2008.
-
- World Health Organization. Iron deficiency anaemia assessment, prevention, and control: a guide for programme managers. Geneva: World Health Organization, 2001.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
