Quantitative assessments of traumatic axonal injury in human brain: concordance of microdialysis and advanced MRI
- PMID: 26084657
- PMCID: PMC4840950
- DOI: 10.1093/brain/awv152
Quantitative assessments of traumatic axonal injury in human brain: concordance of microdialysis and advanced MRI
Abstract
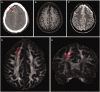
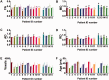
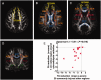
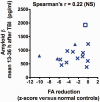
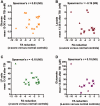
Axonal injury is a major contributor to adverse outcomes following brain trauma. However, the extent of axonal injury cannot currently be assessed reliably in living humans. Here, we used two experimental methods with distinct noise sources and limitations in the same cohort of 15 patients with severe traumatic brain injury to assess axonal injury. One hundred kilodalton cut-off microdialysis catheters were implanted at a median time of 17 h (13-29 h) after injury in normal appearing (on computed tomography scan) frontal white matter in all patients, and samples were collected for at least 72 h. Multiple analytes, such as the metabolic markers glucose, lactate, pyruvate, glutamate and tau and amyloid-β proteins, were measured every 1-2 h in the microdialysis samples. Diffusion tensor magnetic resonance imaging scans at 3 T were performed 2-9 weeks after injury in 11 patients. Stability of diffusion tensor imaging findings was verified by repeat scans 1-3 years later in seven patients. An additional four patients were scanned only at 1-3 years after injury. Imaging abnormalities were assessed based on comparisons with five healthy control subjects for each patient, matched by age and sex (32 controls in total). No safety concerns arose during either microdialysis or scanning. We found that acute microdialysis measurements of the axonal cytoskeletal protein tau in the brain extracellular space correlated well with diffusion tensor magnetic resonance imaging-based measurements of reduced brain white matter integrity in the 1-cm radius white matter-masked region near the microdialysis catheter insertion sites. Specifically, we found a significant inverse correlation between microdialysis measured levels of tau 13-36 h after injury and anisotropy reductions in comparison with healthy controls (Spearman's r = -0.64, P = 0.006). Anisotropy reductions near microdialysis catheter insertion sites were highly correlated with reductions in multiple additional white matter regions. We interpret this result to mean that both microdialysis and diffusion tensor magnetic resonance imaging accurately reflect the same pathophysiological process: traumatic axonal injury. This cross-validation increases confidence in both methods for the clinical assessment of axonal injury. However, neither microdialysis nor diffusion tensor magnetic resonance imaging have been validated versus post-mortem histology in humans. Furthermore, future work will be required to determine the prognostic significance of these assessments of traumatic axonal injury when combined with other clinical and radiological measures.
Keywords: diffusion tensor imaging; microdialysis; tau; traumatic axonal injury; traumatic brain injury.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology 1989; 15: 49–59. - PubMed
-
- Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 1982; 12: 557–63. - PubMed
-
- Adams JH, Jennett B, Murray LS, Teasdale GM, Gennarelli TA, Graham DI. Neuropathological findings in disabled survivors of a head injury. J Neurotrauma 2011; 28: 701–9. - PubMed
-
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008; 34: 51–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

